Isotopes are atoms of the same element, that is, have the same number of protons in their nucleus, that differ in the number of neutrons present in their nucleus. Some isotopes that have too many nucleons tend to be unstable and decay by releasing radiation. Hence they are known as radioactive.
For example carbon has three naturally occuring isotopes, 12C which is the most abundant, 13C and 14C. The isotopes 12C and 13C are stable in nature but 14C is radioactive. A radioactive isotope is said to decay by emitting radiation. Any one of three forms of radiation is emitted:
- beta particle (an electron emitted from the breakdown of a neutron)
- alpha particle (helium nucleus)
- gamma rays.(high energy radiation similar to X-rays but higher in energy)
Isotopes of the same element behave in an identical manner during chemical reactions.
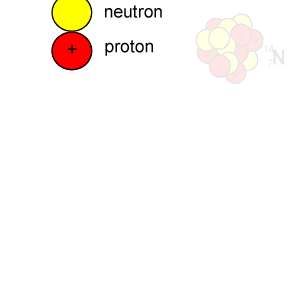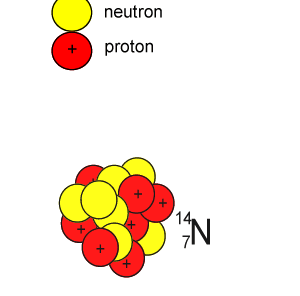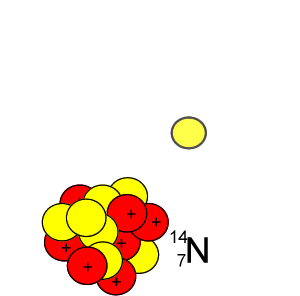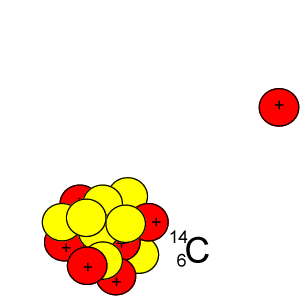
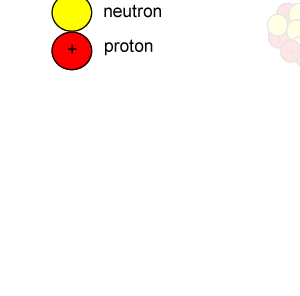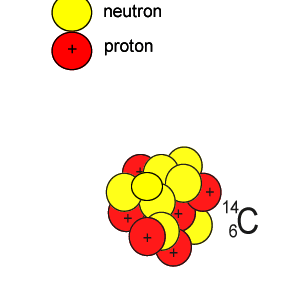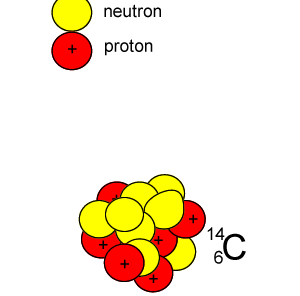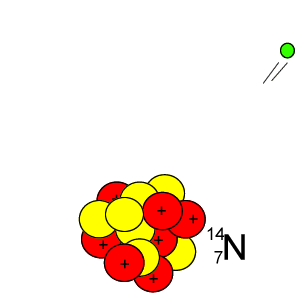
Lets take the 14C isotope. It is used in carbon dating and has a half-life of 5730 years. 14C is formed when high energy neutrons from the sun bombard 14N removing a proton according to the equation below.
10n + 147N => 11H + 146C
Every 5730 years half of the 14C present will decay by releasing a beta particle according to the equation below.
146C => 11-e + 147N
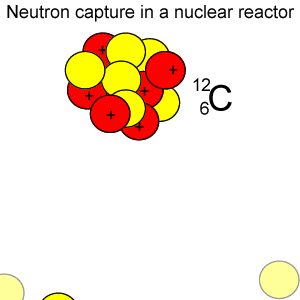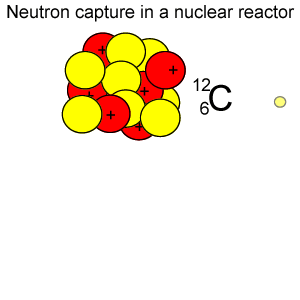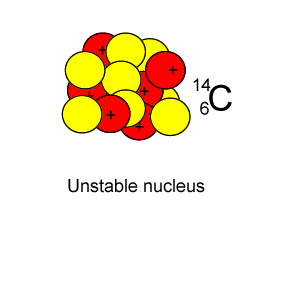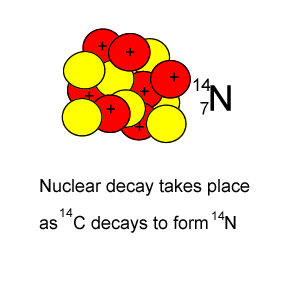
Radioactive isotopes are commonly created in nuclear reactors where neutrons are absorbed in the nucleus of atoms, as shown on the right.
Radioactive isotopes have a wide use in industry. In medicine they are used to fight cancer and to monitor the flow of nutrients through the body.