Greenhouse Effect
Gas emission management
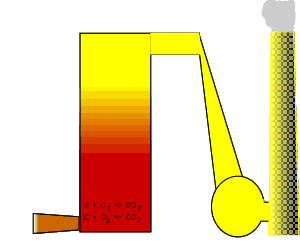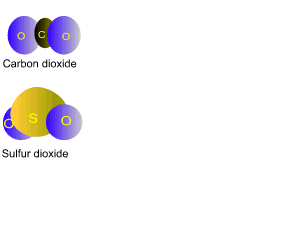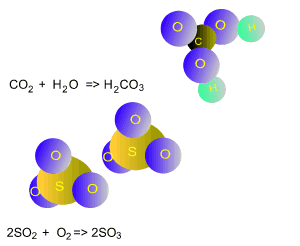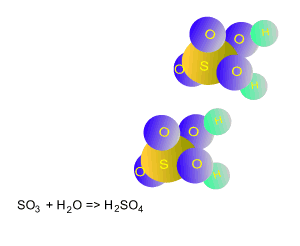
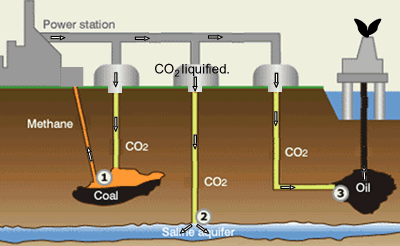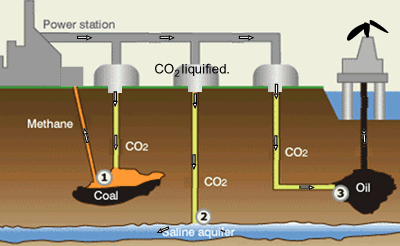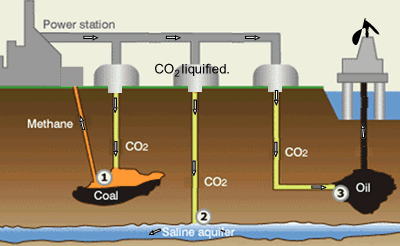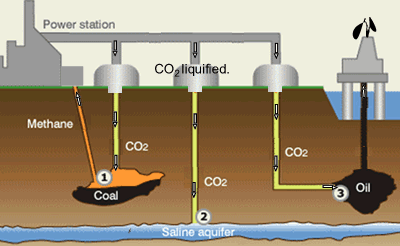
Suggestions of controlling the output of carbon dioxide are to capture
it and liquefy the gas. Once in liquid form carbon dioxide can be pumped
into underground water and used to assist in the extraction of oil and
methane gas, as shown on the right.
Step 1. CO2 is pumped into coal fields to displace methane
gas which is then captured and used as a fuel.
Step 2. CO2 can be pumped into and stored underground salt
water reservoirs.
Step 3. CO2is pumped into oil fields to help maintain pressure
and drive the oil upwards. Liquid CO2 is also used as solvent
to dissolve residual oil. The solution is collected at the surface where
the CO2 separated from the oil and contained.
We can also use disused coal and oil fields to safely store large quantities
of carbon dioxide. Ocean sequestration is also an option.
Carbon dioxide can also be removed by scrubbers. A system consisting of a filter that sucks in fumes through a sodium hydroxide solution. Carbon dioxide reacts with the sodium hydroxide to form sodium carbonate, according to the equation below.
CO2(aq) + 2NaOH(aq) => Na2CO3(aq) + H2O(l)
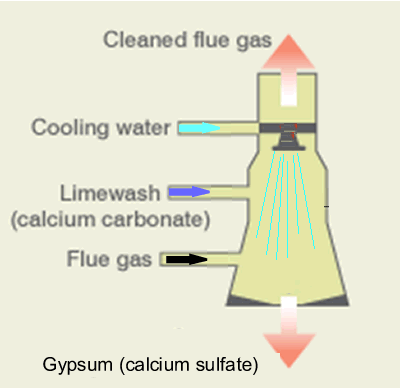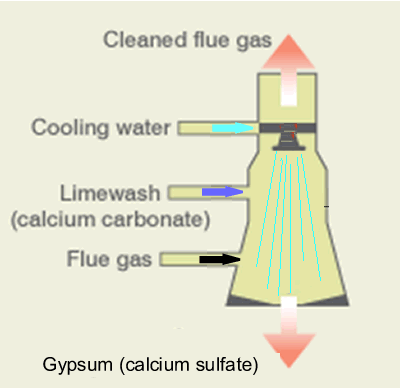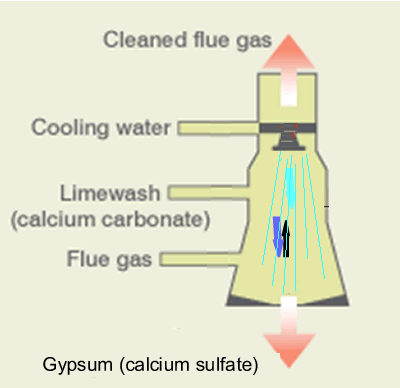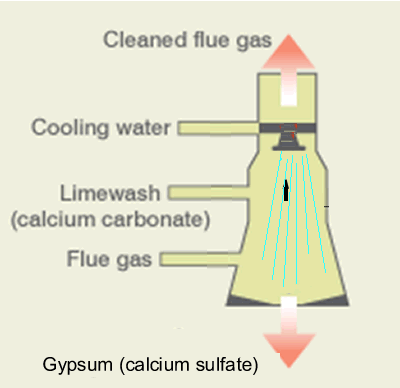
A scrubber is also used to remove sulfur dioxide from fumes. A high pressure liquid is injected into the scrubber where the reaction with sulfur dioxide produces calcium sulfate. Calcium sulfate is then removed from the scrubber and sold to industry. The scrubber provides a large surface area for mass reaction of gas, and the reactions can be performed using limestone, calcium hydroxide, or magnesium hydroxide according tot he equations below.
CaCO 3(s) + SO 2(g) => CaSO3(s) + CO2(g)
Ca(OH)2(s) + SO2(g) => CaSO3(s) + H 2O(l)
Mg(OH)2(s) + SO2(g)
=> MgSO3(s) + H 2O(l)
The calcium sulfite is reacted further to produce calcium sulfate.
2CaSO3(s) + O2(g) + H 2O(l) => 2CaSO4:2H2 O (s)
How does sulfur dioxide react with atmospheric water?
How does sulfur dioxide indirectly contribute to global warming?
Give one use in industry for gypsum (calcium sulfate)
As carbon dioxide is bubbled through lime water (Ca(OH)2) a white solid is seen forming, as pictured on the right.
What is this solid?
Write a chemical equation for this reaction.

Senior chemistry question.
Why is oil not soluble in water but soluble in carbon dioxide? Hint CO2 is a symmetrical molecule.