Greenhouse Effect
CFCs and the ozone layer
Ozone (O3) is our protective layer from Sun's damaging and often lethal UV radiation . Ozone is also a pollutant that irritates the respiratory system. Near the surface of the earth it is formed as a result of car pollution and is present in photochemical smog.

However, in the upper regions of our stratosphere it is a life saving gas that is formed when oxygen molecules are split into oxygen atoms by UV radiation which then attach to oxygen molecules to create ozone. This reaction is shown below and in the animation on the right.
Step 1. Oxygen molecules are split by UV radiation.
O2(g) UV> 2O(g)
Step 2. The free oxygen atoms bond to oxygen molecules to form ozone.
O2(g) + O(g) => O3(g)

Ozone is a very unstable molecule that readily breaks apart to release an oxygen atom and an oxygen molecule in the presence of free atoms such as bromine(Br), and chlorine(Cl).
O3(g) + X(g) => XO(g) + O2(g) ( where X may be Br or Cl)
Although such free atoms are found naturally their concentration in the stratosphere has increased significantly due to human intervention. CFCs were used extensively in aerosols, air conditioners, refrigerators, and for solvents. Two major types of CFCs are trichlorofluorocarbon (CFCl3) and dichlorodifluoromethane (CF2Cl2), pictured on the right. Trichlorofluorocarbon is used in aerosols, while dichlorodifluoromethane is used as a coolant.
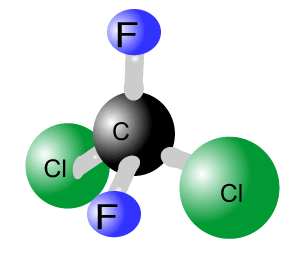
CFCs are very stable and inert compounds that were largely created to replace earlier coolants which tended to cause compressor explosions. This group of compounds is relatively harmless and does not react readily with any other compounds. When in the troposphere these chemicals are indestructible and insoluble in water. We now know that their stability is one of the properties that makes this class of compounds so dangerous. CFCs remain in the atmosphere and over many years find their way to the stratosphere where ozone is prevalent and this is where the damage starts.
CFCs act as catalysts splitting ozone molecules apart. One CFC molecule can continue to split thousands of ozone molecules. Because they are so stable they will persist for many years. Even if we stopped using CFCs today there would be enough molecules in the atmosphere to continue the destruction far into the future. Below is an explanation of the chain of events.
Step 1 In the stratosphere, high energy ultraviolet radiation causes the CFC molecules to break down releasing a chlorine atom(Cl), pictured in green on the right. This chlorine atom acts as a catalyst in the destruction of ozone. Chlorine, like all catalysts, remains unchanged after the reaction and can proceed to destroy thousands more ozone molecules.
Step 2. The chlorine atom initially reacts with the unstable ozone (O3) to form chlorine monoxide (ClO).
Cl + O3 => ClO + O2
Step 3 The chlorine monoxide now reacts with an oxygen atom to produce a molecule of oxygen and to regenerate the atom of chlorine. The regenerated atom of chlorine is free to start a new cycle.
ClO + O => Cl + O2
How does depletion of the ozone layer effect global warming?
Indirectly, depletion of the
ozone layer allows more UV radiation to strike the surface of the planet
and in doing so destroying tiny, microscopic marine plant life called
phytoplankton that float just beneath the surface of the water. So numerous
are these tiny plant forms that they often turn the water green, brown,
or reddish. Phytoplankton utilise dissolved carbon dioxide in photosynthesis
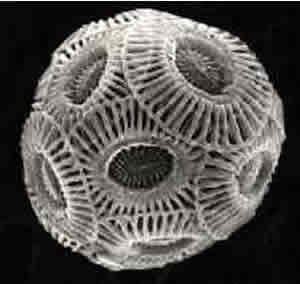
and in forming calcium carbonate skeletons, as pictured on the right.
When you consider that 67% of all photosynthesis that occurs on earth
takes place in the oceans destruction of the phytoplankton is similar
to deforestation. With less phytoplankton to remove dissolved carbon dioxide
the result is pretty clear.