Partial pressure
(Dalton's Law)
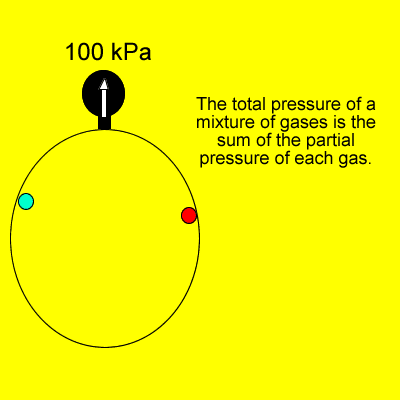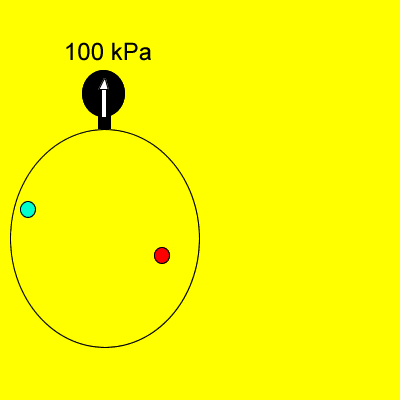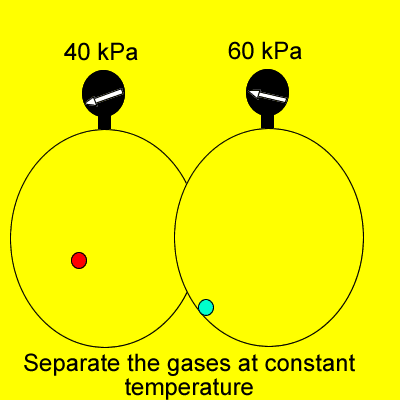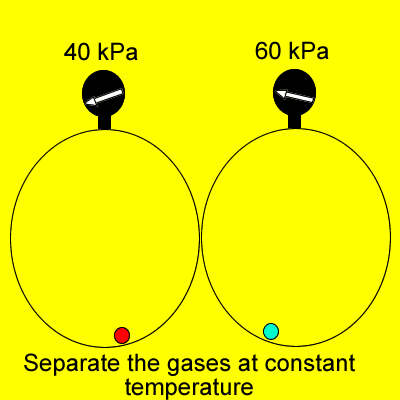
Dalton's Law states that
"Provided the gases do not react, the total pressure of a gas
mixture is the sum of the individual partial pressures of each gas".
|
Partial pressure (Dalton's Law) |
|
|
Dalton's Law states that
|
|

|
In the animation on the left, the total pressure of 100kPa is the sum of the individual pressures of each gas. |