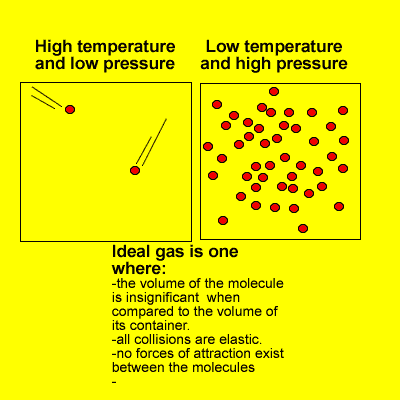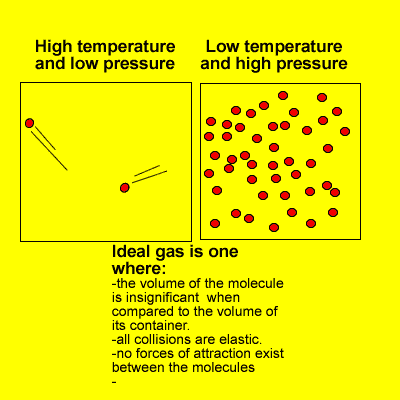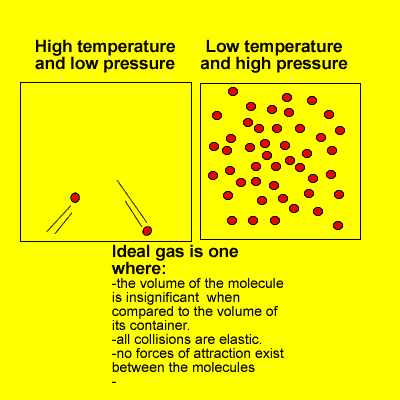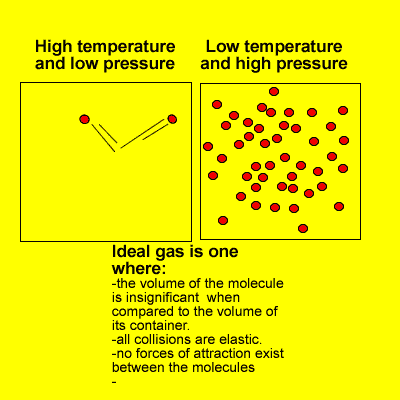
Basic points of the kinetic theory of gases
1) The volume
of gas molecules is negligible compared to the volume of space in which
they move.
2) Gas molecules move in a straight line between frequent collisions with
the walls of the container and themselves.
3) All collisions are elastic, i.e. no energy is lost.
4) There are negligible forces acting between molecules.
5) The average kinetic energy of
the gas molecules is directly proportional to the temperature.
Many of the properties of gases can be explained in terms of the kinetic theory. The kinetic theory describes the behaviour of an ideal gas.
At high pressures and low temperatures attractive forces take over and the volume of gas molecules is significant when compared to the volume of space in which they move.
However, at high temperatures and low pressures real gases do behave as ideal gases meeting every requirement of the kinetic theory.
Can an ideal gas be liquefied?
Explain
Why does a real gas behave as an ideal gas at high temperatures and low
pressures?
Compare and state the differences between a real gas and an ideal gas.
Explain why gases are easily compressed.
What is meant by the average kinetic energy of gas molecules
at a given temperature?
Jonathon pumps his bicycle tyres using a hand pump. The nozzle gets very
hot as gas is compressed into it. What part of the kinetic theory is not
obeyed by the compressed air?
Solutions