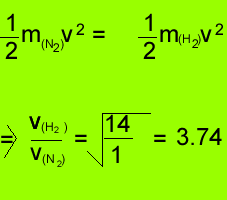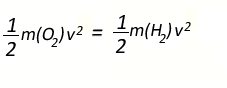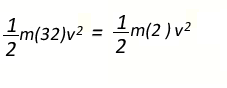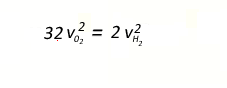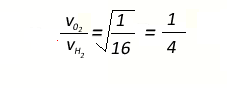The average kinetic energy
of oxygen molecules and hydrogen molecules will be the same at any particular
temperature. If we keep in mind that the formula for kinetic energy
is 0.5mv2 it stands to reason that the lighter molecules(hydrogen)
will have a greater speed than the oxygen molecules.
Since the average kinetic energy of oxygen and hydrogen are equal we
can write the expression below.
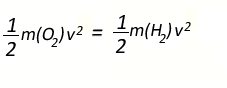
However the
molecular mass of oxygen is 32 and that of hydrogen is 2. So we can
rewrite the above expression
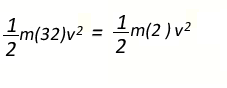
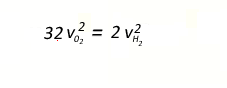
Transforming
the formula we obtain the expression below which gives the ratio of
the average velocities of oxygen and hydrogen molecules
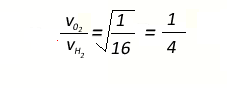
The average velocity of hydrogen
molecules is 4 times greater than that of oxygen molecules.
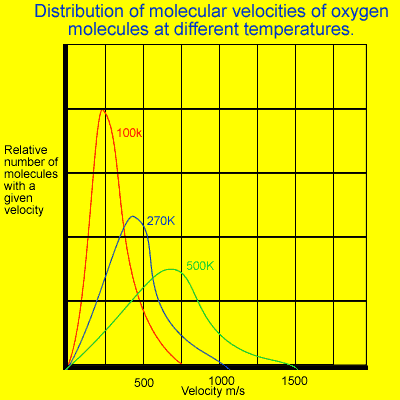
It is important
to note that although the average kinetic energy of all gases is the
same at a particular temperature, the average velocity of the molecules
is not. The heavier molecules tend to travel slower relative to the
lighter ones.