Carbon dioxide
When vinegar (weak acid) is added to sodium carbonate powder the gas known as carbon dioxide is produced as shown on the video on the right.
View the video on the right.
1) Carbon dioxie is formed when and react.
2) Carbon dioxide
3) Carbon dioxide
Click for a more detailed view of the carbon cycle.
Carbon circulates through the biosphere in the form of carbon compounds such as carbon dioxide. A simple overview is shown above.
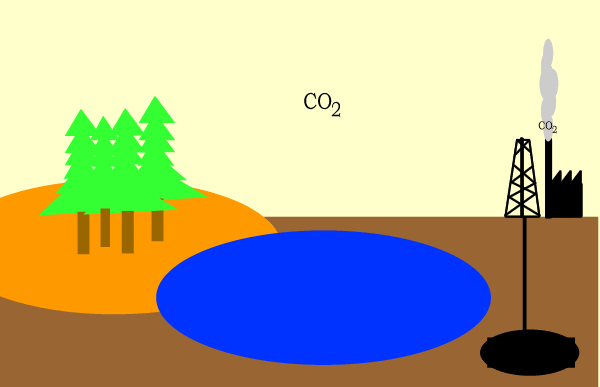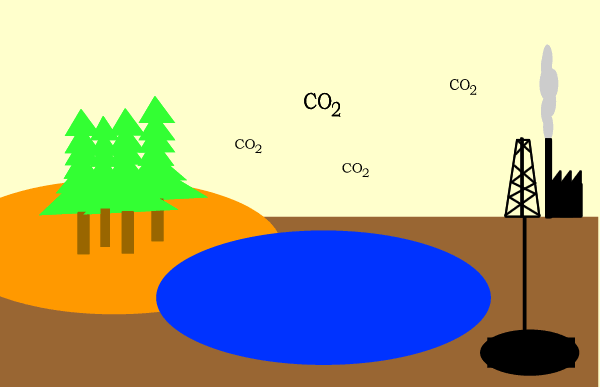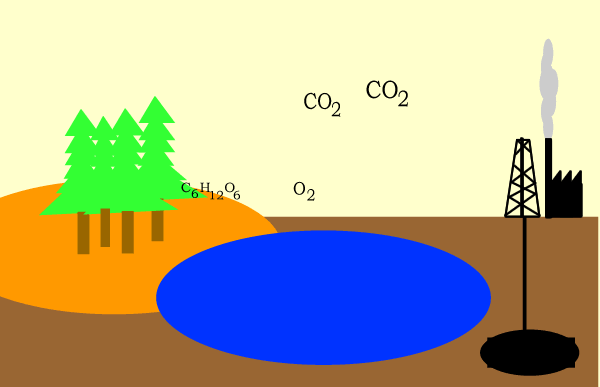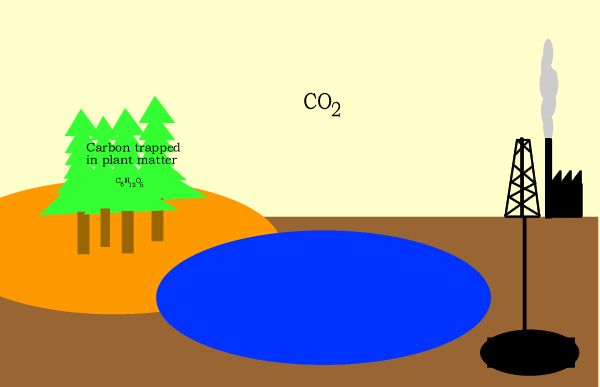
Carbon dioxide is used as a raw material in the making of glucose during the process of photosynthesis as shown below.
6CO2(g) + 6H2O(l) => C6H12O6(aq) + 6O2(g)
The glucose is then converted to starch and cellulose and stored in the
plant.
Carbon dioxide is produced during cellular respiration according to the reaction below.
C6H12O6(aq) + 6O2(g) => 6CO2(g) + 6H2O(l)
It is also produced in abundance by industry and motor vehicles when fossil fuels are burnt. The general reaction is given below.
Hydrocarbons + O2(g) => CO2(g) + H2O(g)
Carbon dioxide is used to carbonate soft drinks. When you open a bottle of soft drink, bubbles of carbon dioxide rise to the surface. Click to see a 120 kb video. Some of the dissolved carbon dioxide reacts with water to produce carbonic acid according to the reaction below. For this reason carbon dioxide is classified as an acidic oxide.
H2O(l)
+ CO2(l) => H2CO3(aq)
Carbonic acid is a very weak acid and has a pleasant taste.

