Trans fats
Article modified from ChemMatters December 2007 by Doris R. Kimbrough

Before we look at trans fats we will take a look at normal fats. Fats and oils, fats which are liquid at room temperature, are produced by plants and animals as an energy store. In animals fat also serves as an insulator.
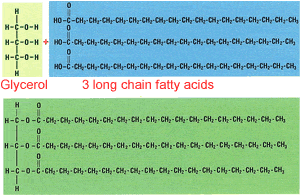
All fats and oils have a similar structure, three long chain fatty acids, between 12 to 20 carbons in length, attached to a glycerol molecule, as shown on the right. Fats and oils are referred to as triglycerides.
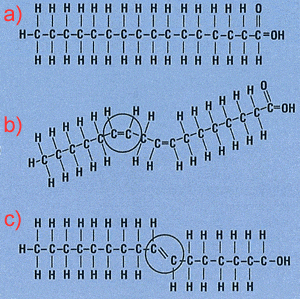
Saturated fats are commonly produced by mammals and are characterised by having only single carbon-carbon bonds. This allows carbons to bond with the maximum number of hydrogen atoms possible and hence the molecule is said to be saturated with hydrogens. Consider molecule a) on the right. This represents a saturated fatty acid while the molecule labelled c) represents a unsaturated fatty acid. The molecule labelled b) is said to be poly-unsaturated as it has more than one double bond.
Plants and some fish have usaturated fats.
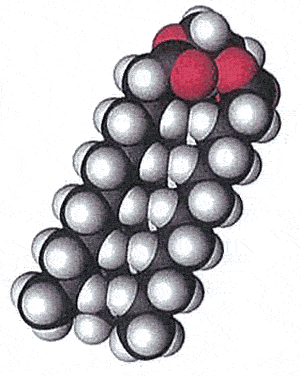
Saturated fats have a linear three-dimensional shape as pictured on the right by the space filling model. This allows the fat molecules to pack in close together. Saturated fats are found as solids at room temperature. Think of butter, lard and the fat you cut off from a steak.
Fat molecules are non-polar and rely on weak forces of attraction called van der Waals forces to bond together into a solid. The strength of these forces is dependent on the size of the molecule and the distance apart from each other. Since saturated fats pack close to their neighbours van der Walls forces are increased.