Trans and cis
Article modified from ChemMatters December 2007 by Doris R. Kimbrough

Unsaturated fats are healthier than saturated with the latest studies indicating that the type of fat one eats directly affects the individual's health.
Many foodmakers advertise their products as healthy alternatives by emphasising the presence of unsaturated fats in their foods.
However, one must realise that all fats have a great deal of energy and a diet high in fat will have adverse health effects.
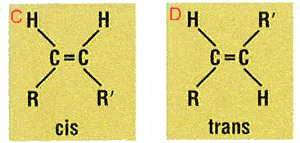
Most naturally occurring fatty acids, however, have the cis configuration and are found nuts, fish and in vegetable oils. It’s usually when the fats are hydrogenated to modify their behaviour that the trans forms are produced.
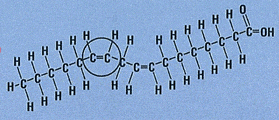
Molecules with cis double bonds are more likely to react with oxygen in the air, than molecules with trans double bonds, to form shorter chain molecules with unpleasant smells. Food companies are faced with a dilemma. If they use healthier unsaturated fats they face the risk of the food spoiling, becoming rancid.
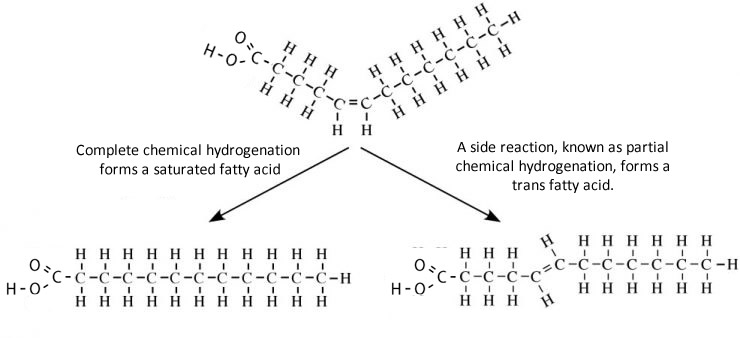
Hydrogenation is a process used by food makers to convert a cis to a trans double bond. During hydrogenation a cis unsaturated fat is heated at high pressure and in the presence of hydrogen gas and a nickel catalyst yields a trans double bond.
Until recently partially hydrogenated fatty acids were thought to be as healthy as the cis unsaturated fatty acids they were made from. But recently links to various serious health risks, that include cancer, have been suggested. One prevalent theory is that a fat digesting enzyme, known as lipase, is unable, to breakdown trans-fatty acids and hence these build up in the blood causing significant damage and blockage to blood vessels.
Trans fats have a semisolid property much liked by consumers. Margarine was advertised as a healthy alternative to butter and more convenient to use because the partially hydrogenated fatty acids made margarine spread easily on bread.
1) What is partial hydrogenation?