Equilibrium
expression
Print out this worksheet and write the equilibrium expression for each equation below.
|
Equation
|
Equation
|
Equilibrium
expression
|
|
1)
|
|
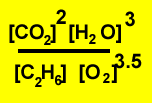 |
|
2)
|
|
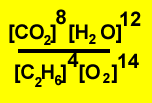 |
|
3)
|
|
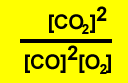 |
|
4)
|
|
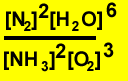 |
|
5)
|
|
 |
|
6)
|
|
 |
Equation 4 has an equilibrium
constant at an unknown temperature of 2 X 10-15
/
Explain the significance of this value.
The equilibrium expression is given above. A large value obviously means a great amount of product at equilibrium as compared to the reactants. The reaction is therefore said to favour the formation of products. However the value of "K" is extremely small 2 X 10-15 and as such, little product is formed. So at this unknown temperature the reaction does NOT favour the formation of product.