Coal seam gas (CSG) is reported to be a clean alternative to coal and other natural fuels. It is almost pure methane, with a small percentage of carbon dioxide which is removed before going to the end user. CSG is formed in amongst coal deposits by microbial action. It is kept in coal seams under pressure by water. A bore is drilled and the gas along with the water recovered and separated as shown on the right.
It is free of sulfur, nitrogen and creates no airborne particles when burnt. In essence it is a clean fuel from this perspective. But like any other fossil fuel it will burn in air to create carbon dioxide according to the equation below.
CH4 + 2O2 =>2 H2O + CO2
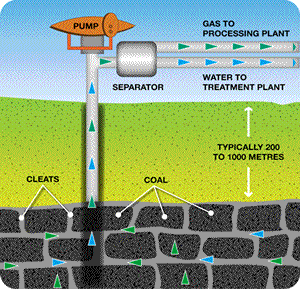
Picture from. www.aplng.com.au/whatiscsg.html
The water that flows out with the methane gas is presently deposited in above ground evaporation ponds. This water is heavily saline, contaminated with metals and toxic hydrocarbons of various types from the coal seams. Releasing this water untreated into the natural environment is not an option. So the evaporating basins are a cheap alternative. A more costly alternative is to pump the water back underground deep enough as to not contaminate the underground water table. But what happens when the methane reserves are exhausted and mining ceases? What is left are many hectares of dangerous sludge in the evaporating basins that must be removed. It has been suggested that treated water be used for stock and irrigation. Some possible risks include: - proliferation of disease carryng mosquitoes that thrive in highly saline water. When it comes to clean fossil fuels there is more to consider than sulfur content. |
|
| Why is it important for fossil fuels to have a low sulfur content? | |
| What is acid rain? |