Inter molecular
bonding
Between the molecules.
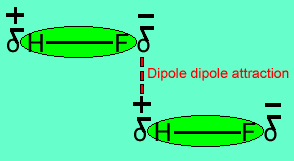
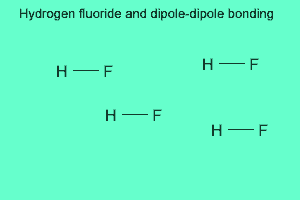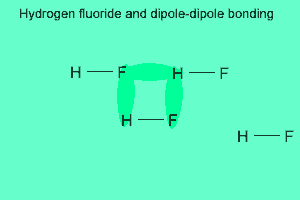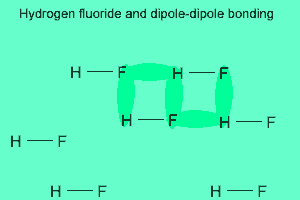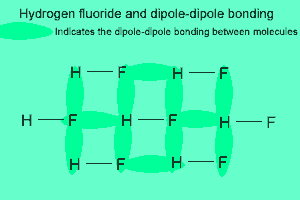
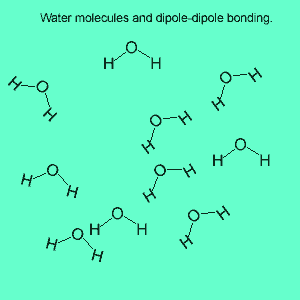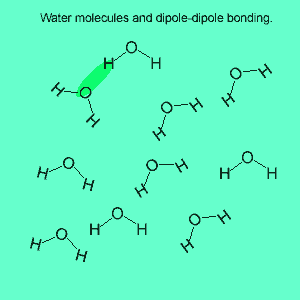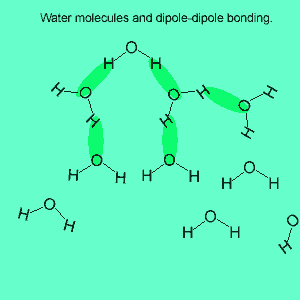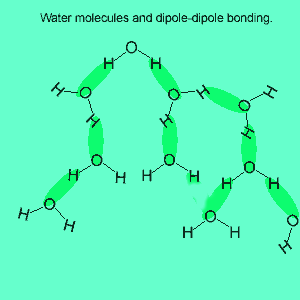
The image above shows a polar molecule (HF) and the way the poles of each molecule interact to bring about intermolecular bonding called dipole-dipole bonding..
Uneven sharing of electrons in a covalent bond is one of the factors that gives rise to polar molecules. The other factor is a lack of symmetry in the molecule, but we will discuss this a little later. A polar molecule is one that has small charges (not single charges as in ionic bond) on opposite ends. Polar molecules attract each other via the charges on each end. Attraction of opposite ends of polar molecules is called dipole-dipole bonding. This form of intermolecular bonding is extremely weak when compared to ionic or metallic bonding. There is a strong form of dipole-dipole bonding known as hydrogen bonding which occurs when hydrogen is bonded to an oxygen, nitrogen or fluorine atom. It is strong enough to allow water to exist as a liquid at room temperature. This strong form of intermolecular bonding is the reason why a small molecule such as water exists as a liquid at room temperature and not as a gas.
Notice the animation on the right of HF undergoing hydrogen bonding. Note how the opposite ends of the molecules align themselves so that the small negative and positive charges attract.
.