|
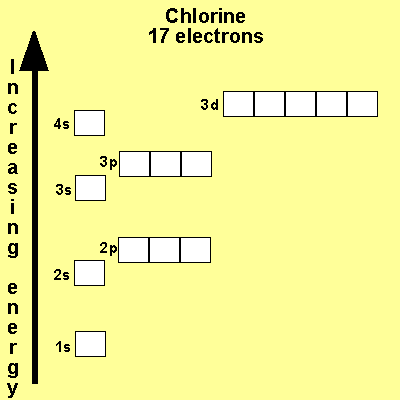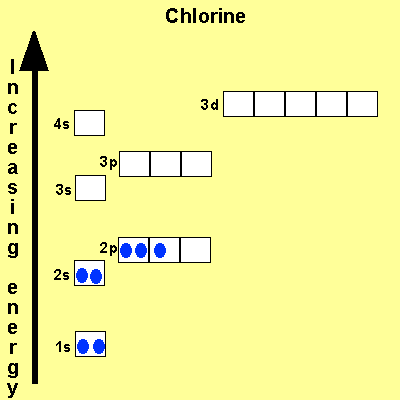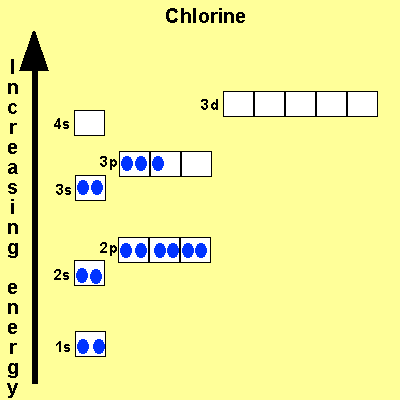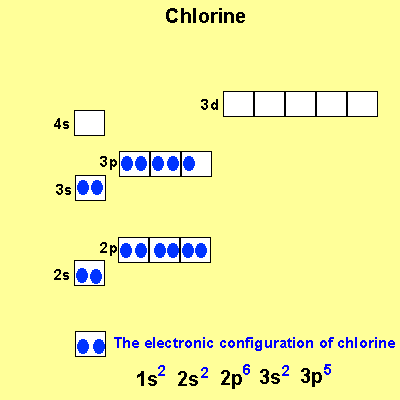
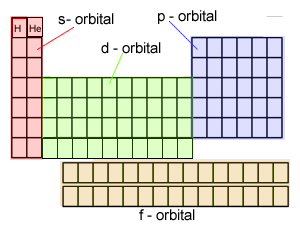
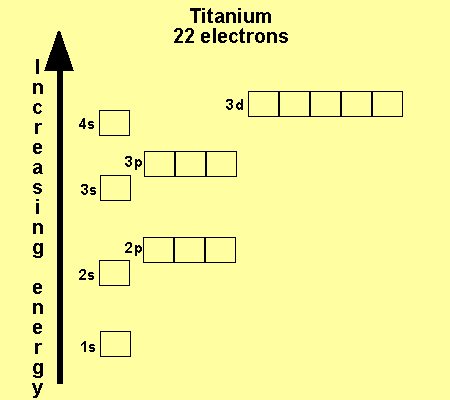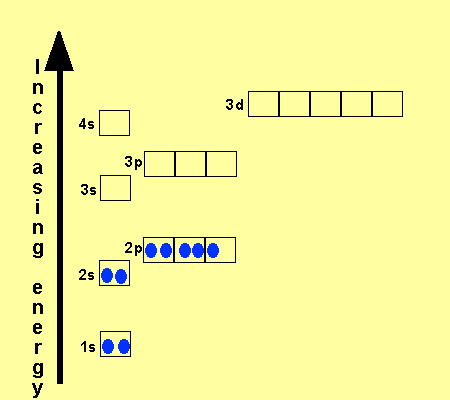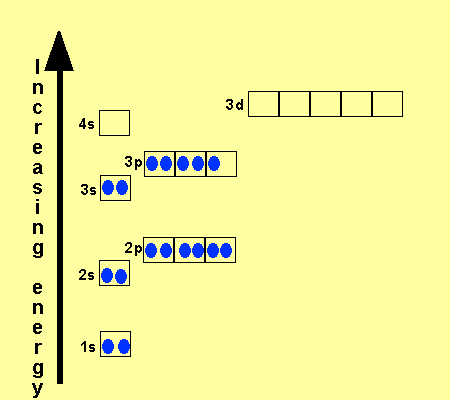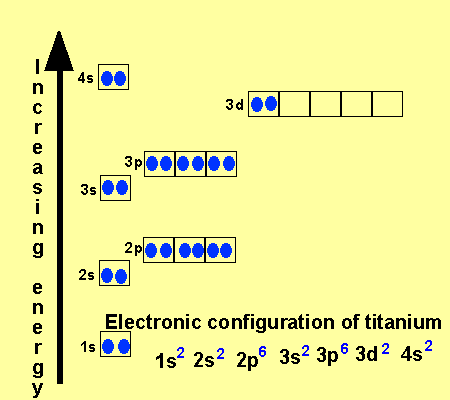
The electronic configuration
of Cl is
1s2,
2s2, 2p6, 3s2, 3p5
Na is
1s2, 2s2, 2p6, 3s1
Cu is
1s2, 2s2, 2p6, 3s2,
3p6, 3d9, 4s2
Co is
1s2, 2s2, 2p6, 3s2,
3p6, 3d7, 4s2
Fe is
1s2, 2s2, 2p6, 3s2,
3p6, 3d6, 4s2
|
|
|
a) Nickel 1s2,
2s2, 2p6, 3s2, 3p6, 3d8,
4s2
b) Aluminium 1s2, 2s2, 2p6,
3s1, 3p2
Aluminium is excited because the 3s subshell is not full while the
3p contains electrons.
c) Element "X" 1s2, 2s2, 2p6,
3s2, 3p5, 4s2
"X"
is excited because the 3p subshell is not full while the 4s contains
electrons.
d) Element "Z" 1s2, 2s2, 2p6,
3s2, 3p6, 3d5, 4s1
"Z" is excited
because the 4s subshell is not full while the 3d contains electrons.
e) Element "Y" 1s2, 2s2, 2p6,
3s2, 3p10, 3d2, 4s1 "Y"
is excited because the 4s subshell is not full while the 3d contains
electrons.
3) Aluminium has the atomic
number 13 and a mass number of 27. Write the electronic configuration
of the aluminium (Al3+) ion.
The aluminium ion has 10 electrons and must therefore have the electronic
configuration shown below.
1s2, 2s2, 2p6
4) Write the electronic
configurations of the following ions.
a) Cr2+
1s2, 2s2, 2p6, 3s2,
3p6, 3d2, 4s2
b) Fe3+
1s2, 2s2, 2p6, 3s2,
3p6, 3d3, 4s2
c) Zn2+
1s2, 2s2, 2p6, 3s2,
3p6, 3d8, 4s2
|