|
The electronic configuration of Cl is 1s2, 2s2, 2p6, 3s2, 3p5 Na is Cu is Co is Fe is |
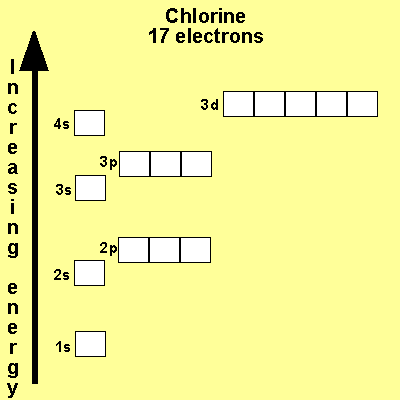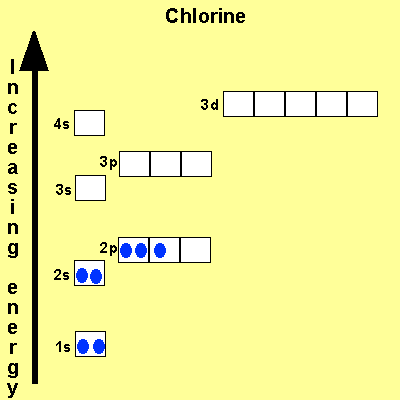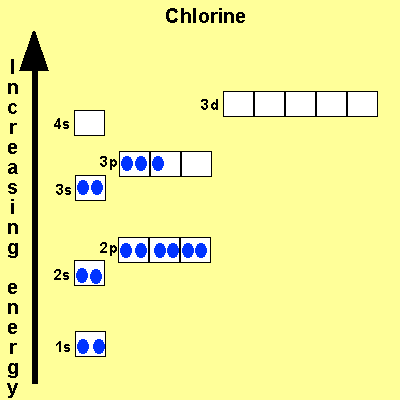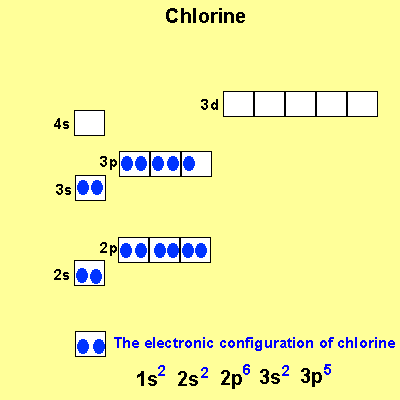 |
|
a) Nickel 1s2,
2s2, 2p6, 3s2, 3p6, 3d8,
4s2 3) Aluminium has the atomic
number 13 and a mass number of 27. Write the electronic configuration
of the aluminium (Al3+) ion. 4) Write the electronic
configurations of the following ions. |
|
Click to hide the solution