Adapted from an article in NewScientist September 2015 "Modern-day alchemy is putting the periodic table under pressure"
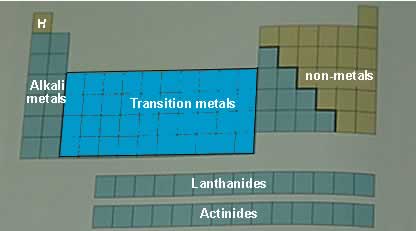
The modern day periodic table contains all the known elements. They are placed in unique positions on the table according to their electronic configuration. The table is divided into rows (periods) and columns (groups). It is further divided into different regions such as those shown in the diagram above.
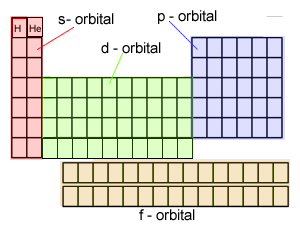
It is the position of the outer shell electrons, known as valence electrons, that is critical to the chemical behaviour of elements and so the elements are placed in regions on the table that correspond to the location and number of their outer shell electrons. The coloured squares, on the right, depict the location of the valence electrons. Click on this link to see how the electronic configuration of each element is used to place them in the periodic table.
In 1869, Dimitri Mendelev, a Russian chemist, organised the periodic table according to the atomic mass of elements. He quickly noticed that certain chemical behaviours were repeated as the atomic mass increased. He organised elements with similar chemical properties in groups and elements with increasing atomic mass in rows. This showed that patterns begin to emerge in the properties of the elements and used those patterns to predict the existence of, as yet, undiscovered elements.
We now know that chemical properties are due to electronic configuration and not atomic mass. The modern day periodic table has elements arranged according to their atomic number. We have reactive metals on the left, groups 1 and 2 and non-metals on the right. In the middle we have a group of elements called the transition metals. These physical properties exhibited by the elements are based on normal pressures, 1 atm, and room temperatures.
It was found, almost ninety years ago by J.D.Bernal that any non-metal element of the periodic table will transform, at high enough pressures, into a metallic state. Elements such as hydrogen, helium and oxygen have been shown to be conductors at high enough pressures and temperatures. Exert a pressure similar to that found in Earth's outer core, about 3,500,000 times that of the pressure found on the surface, and sodium , which is normally a soft, highly reactive metal, becomes transparent and something between a semiconductor and an insulator. Oxygen, meanwhile, is a solid metal at similar pressures that can be cooled to become a superconductor. This shows that the physical properties of any element depends on the conditions it exists in.
Under incredible pressure, as exists in the centre of the Sun and planets such as Earth, Jupiter and Saturn, atoms get squashed and electrons squeeze into tighter orbitals, that take up irregular exotic configurations thus forming chemical bonds with
electrons in other atoms in entirely different and unimaginable ways.
Such interesting behaviour of these elements at high pressures can result in new and innovative products that can revolutionise our ability to produce and transmit energy. Calculations suggest that when hydrogen is subjected to such high pressures and temperatures the electron from each atom is squeezed out and floats throughout the solid proton lattice, just like any metal. Unlike a normal metal, however, the electrons in hydrogen metal may be capable of moving unhindered, no resistance, through the lattice thus creating a superconductor. This might even occur at room temperature and atmospheric pressures, much like how diamond structure exists even when the pressure in which it was formed is removed. This has enormous consequences for the transmission of energy along great distances without loss and the production of electric motors that would banish the internal combustion engine to history.
Now, you might think that such states are rare, but when you consider that 99.9% of matter is found within planets and stars, where temperatures are very high and pressure unimaginably high, then the majority of elements have totally different properties than they exhibit at low temperature and pressure.