Elements with the same number of valence(outer shell) electrons are placed in the same group. While elements with electrons in the same valence shell(outer shell) are placed in the same period.
|
|
Group
1
|
Group
2
|
Group
3
|
Group
4
|
Group
5
|
Group
6
|
Group
7
|
Group
8
|
|
Period
1
|
Hydrogen 1s1 |
|
|
|
|
|
|
Helium 1s2 |
|
Period
2
|
Lithium 1s2, 2s1 |
Beryllium 1s2, 2s2 |
Boron 1s2, 2s2, 2p1 |
Carbon 1s2, 2s2, 2p2 |
Nitrogen 1s2, 2s2, 2p3 |
Oxygen 1s2, 2s2, 2p4 |
Fluorine 1s2, 2s2, 2p5 |
Neon 1s2, 2s2, 2p6 |
|
Period
3
|
Sodium 1s2, 2s2, 2p6, 3s1 |
Magnesium 1s2, 2s2, 2p6, 3s2 |
Aluminium 1s2, 2s2, 2p6, 3s2, 3p1 |
Chlorine 1s2, 2s2, 2p6, 3s2, 3p5 |
Notice how the elements in
group 1 have 1 valence electron while all the elements in period two have
energy level 2 as the valence(outer) energy level.
Now lets have a look at some trends. The following trends are obvious
when elements are placed in the periodic table and can be explained using the notion of core charge. Spend a moment to review core charge.
|
Down
a group
|
Across
a period
|
- Atomic size increases |
- Atomic
size decreases |
| -Metallic character increases | - Metallic character decreases |
| - Electronegativity(ability to attract electrons into the valence shell) decreases. As a result reducing(electron giving) strength increases, while oxidising(electron taking) strength decreases. | - Electronegativity increases. As a result reducing(electron giving)
strength decreases, while oxidising(electron taking) strength increases. -Click to see trend across period 3 -Click to see trend across period 4 |
| - First
ionisation energy decreases Click for more information on core charge and first ionisation energy. |
- First ionisation energy increases |
 |
Click to see a more detailed explanation of the above trends.
The oxides of
elements tend to be more acidic as we move across the period.
Sodium oxide reacts with water according to the reaction below to produce
a highly alkaline solution.
2Na(s) + 2H2O(l) => 2NaOH(aq) + H2(g)
Aluminium
oxide(Al2O3) is amphoteric.
It reacts with both acids and bases according to the reactions below.
Al2O3(s) + 6H+(aq) => 2Al3+(aq) + 3H2O(l) (Shows
aluminium oxide acting as a base)
Al2O3(s) + 2OH-(aq) + 3H2O(l) =>
2Al(OH)4-(aq) (Shows
aluminium oxide acting as an acid)
While sulfur trioxide reacts with water to produce a very strong acid
according to the reaction below.
SO3(g) + H2O(l) => H2SO4(aq)
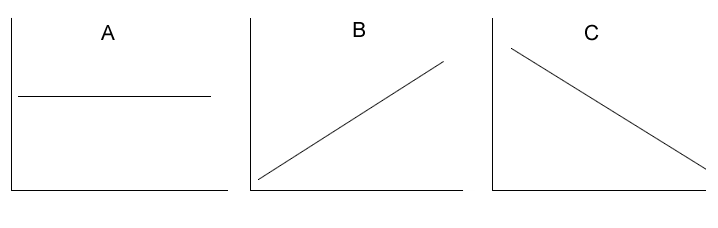
Above are the graphs showing the change in a particular characteristic of an atom.
Which graph best represents the:
i) change in core charge as we move down a group?
ii) change in core charge as we move across a period?
iii) change in first ionisation energy across a period?
iv) change in first ionisation energy down a group?
v) change in
atomic radius across a period?
vi) change in atomic radius down a group?
vii) change in electronegativity down a group?
viii) change in electronegativity across a period?