In 1932 James Chadwick identified the neutron. The particle proposed by Rutherford as having significant mass and no charge. With the discovery of the neutron three subatomic particles were identified that would help explain observations made at the atomic level. One observation was the existence of radioactive variances of the same element.
How could two atoms of the same element have identical chemical properties but one be radioactive and the other not? A British scientist Frederick Soddy who made this observation called varieties of the same element isotopes.
Chadwick was now able to explain the existence of isotopes through his discovery of the neutron. Isotopes of the same element have the same number of protons and electrons but differ in the number of neutrons found in their nucleus.
Before Chadwick's discovery of the neutron in 1932, a Danish scientist by the name of Neils Bohr refined Rutherford's model in 1913 by proposing that electrons:
- orbit the nucleus without losing energy;
- could move only in fixed orbits of specific energies.
Electrons with low energy would orbit closer to the nucleus while electrons with high energy orbit further from the nucleus.

![]()
View the video on the right.
1) The most abundant hydrogen atom is composed of
2) What charge is an electron
3) What charge is a proton?
4) What would happen if an electron orbited the nucleus in a circular path?
5) What did Bohr say about an electron orbiting the nucleus and the energy level it is allowed to have?
6) An electron can occupy orbits that are
7) What hypothesis did Bohr put forward to explain why the electron dose not lose energy and fall into the nucleus?
8) What evidence did Bohr have to support his hypothesis?
9) By assuming the electron can act as both a particle and a wave what were scientists able to explain?
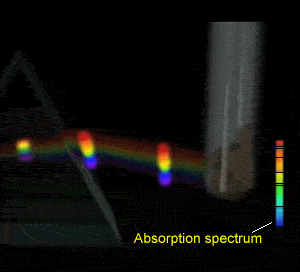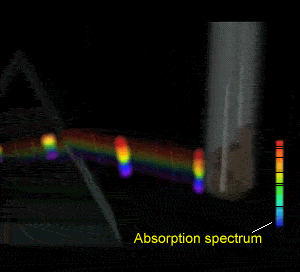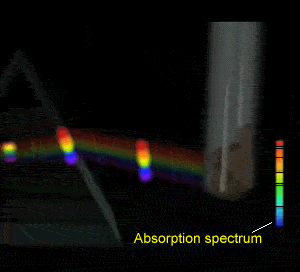
Flame tests revealed emission spectra that Bohr's model could adequately explain. Click to see the flame test of copper.
Electrons could absorb heat energy and jump from their stable ground state (lowest energy possible) to higher energy levels, as shown on the right. The energy released would be emitted as a wavelength of light proportional to the amount of energy it had absorbed. Different colours indicate a different energy jump.
Click here for a detailed explanation of emission spectra.

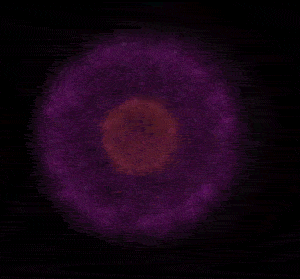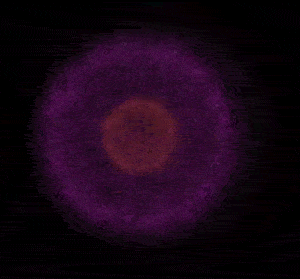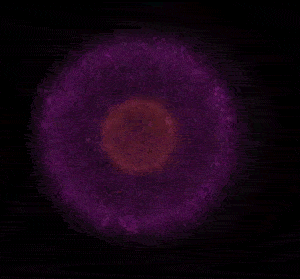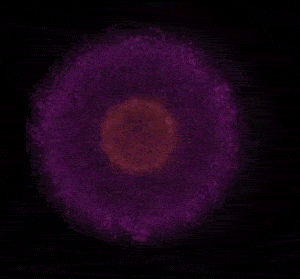
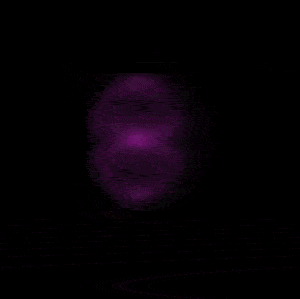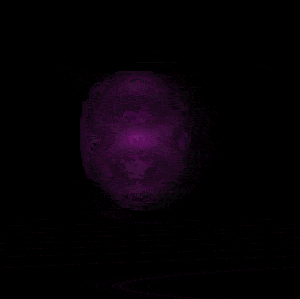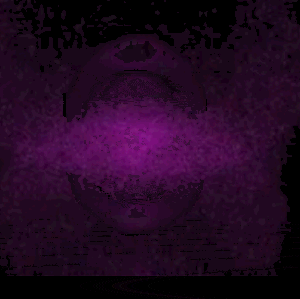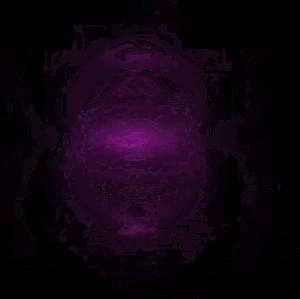
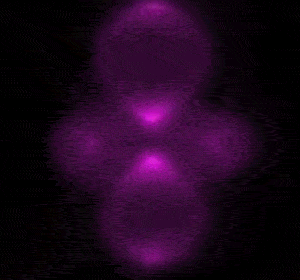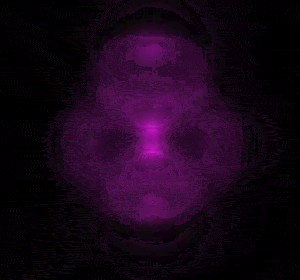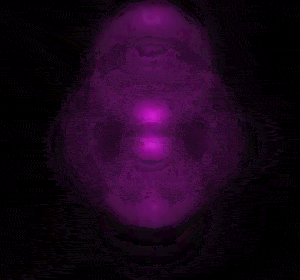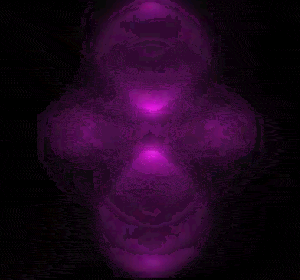
In 1926 Erwin Schrödinger, an Austrian physicist, took the Bohr model one step further. Using mathematical equations Schrödinger described the likelihood of finding an electron in a defined space around the nucleus. This space is known as an orbital and according to the equation has a clearly defined energy level and shape. This atomic model is known as the quantum mechanical model of the atom.
Unlike the Bohr model, the quantum mechanical model does not require electrons to orbit the nucleus in clearly defined paths, but rather, predicts the likelihood of the location of an electron. In other words a region of space, orbital, is designated where an electron is likely to be found. This region is most dense where the electron has a high probability of been located at any particular time. Hence the model is portrayed as a nucleus surrounded by an electron cloud. This model introduced the concept of sub-energy levels. For example, take the second energy level of an atom, it is made up of two sub-energy levels known as the "s" and "p" sub-energy levels.
The "s" sub-energy level
is spherical while the "p" sub-energy level is composed of three dumbell shaped orbitals along the three axis, x, y and z in 3-dimensional space around the nucleus.
But that's enough of that for the moment, orbitals and electronic configuration will be covered later but you can click on the link if you wish to study these now.