Electrons move in regions of space surrounding the nucleus called orbitals. These orbitals can be regarded as being arranged in shells around the nucleus. These shells are major energy levels surrounding the nucleus. Shells are further divided into energy levels of similar energy called subshells. These subshells are given the labels of "s", "p", "d", "f" and "g". Subshells are further composed of orbitals, which, as discussed above, are regions of space around the nucleus in which electrons can be found. The subshells within a shell increase in energy in the following order, s<p<d<f<g.
Orbitals can have 0, 1 or 2 electrons in them. This is known as the Pauli exclusion principle.
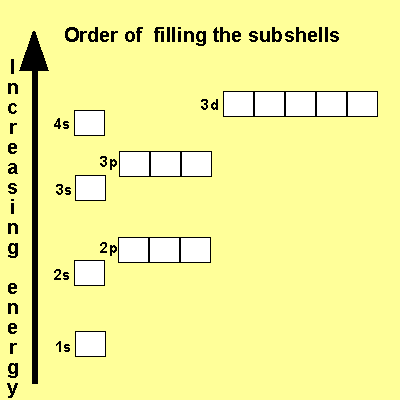
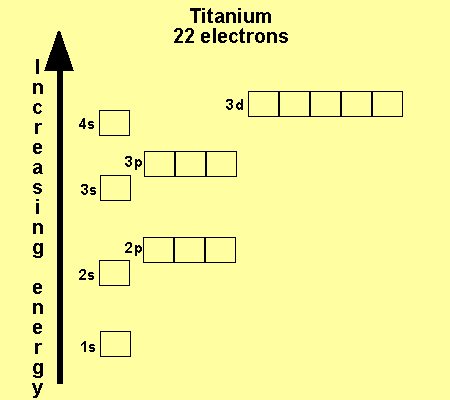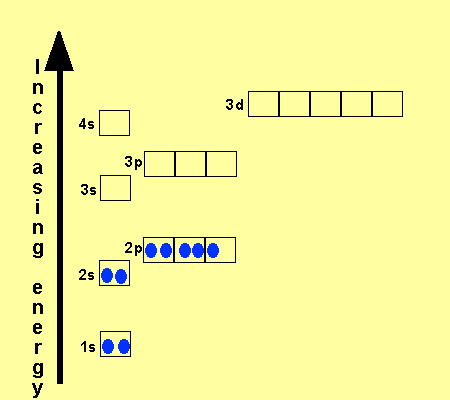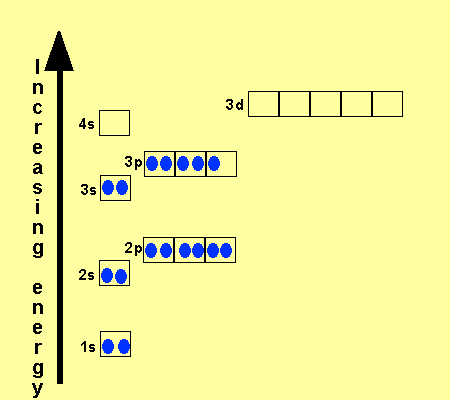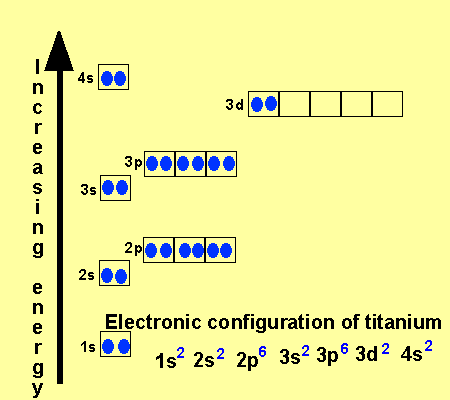
Look at the different energy levels of an atom as pictured above.
The first energy level has one subshell in it called the "s" subshell. This subshell has room for only one orbital and therefore 2 electrons.
The second energy level has room for two subshells, the "s" and "p". Both the "s" and "p" subshells have a total of 4 orbitals and therefore can hold a total of 8 electrons.
A many electron
atom fills its lowest energy shells first before proceeding to higher
energy shells. The order of filling is
1s, 2s, 2p, 3s, 3p, 4s, 3d .
Notice how the 4s subshell is filled before the 3d subshell.
The 3d has more energy than the 4s subshell. Look at the way titanium
fills its subshells below.
Click on each atom to see the configuration
Hydrogen
Helium
Lithium
Berylium
Boron
Carbon
Nitrogen
oxygen
Fluorine
Neon