Foods are great examples of mixtures. Chips are particularly interesting as they contain saturated and unsaturated fats, carbohydrates, such as starch, salt and flavour additives.
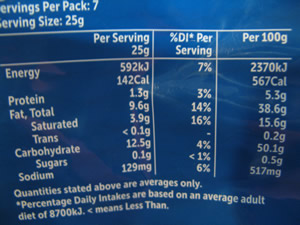
The quantities of each additive are listed on the package, as shown on the right.
In this investigation we will separate the salt and fats from chips, calculate the percentage amount and compare it to the package label.


3) Using a measuring cylinder measure approximately 25 mL of acetone and add it to the evaporating dish with the crushed chips. Use a glass rod to stir for 5 minutes.
Acetone will dissolve the fat.




7) Once the acetone has evaporated weigh the evaporating dish with the fat.
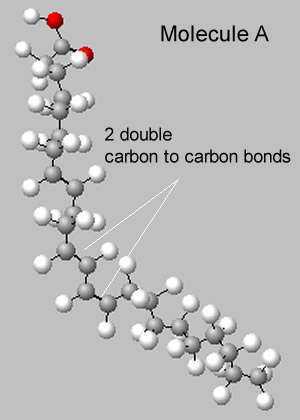
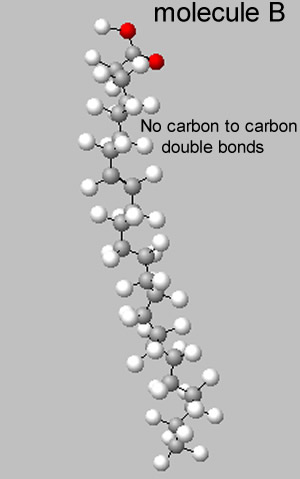
If the fat appears to be solid at room temperature it is most likely a saturated fat.
If it is a liquid it is predominantly an unsaturated fat.
Using the data from the label. shown above, calculate the percentage, by mass, of fat in the chips.
Solution
Using the results shown here calculate the percentage, by mass, of fat in the chips.
Solution
How do the results compare? Give an explanation
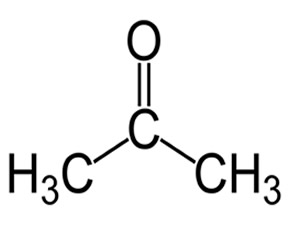
2) Explain why acetone is mildly soluble in water and also able to dissolve fats.
a) Saturated fats.
b) Unsaturated fats.
4) Explain why unsaturated fats would be liquid at room temperature while saturated fats would be solid. Use the two molecules shown on the right in your explanation.
Use the words
- intermolecular bonding
- dispersion forces
- saturated
- unsaturated
- polar
- non-polar