Salt from chips.

Foods are great examples of mixtures. Chips are particularly interesting as they contain saturated and unsaturated fats, carbohydrates, such as starch, salt and flavour additives.
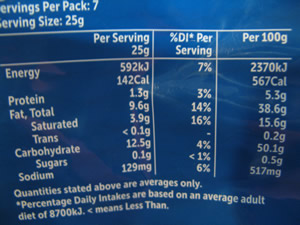
The quantities of each additive are listed on the package, as shown on the right.
In this investigation we will separate the salt and fats from chips, calculate the percentage amount and compare it to the package label.


3) Using a measuring cylinder measure approximately 25 mL of acetone and add it to the evaporating dish with the crushed chips. Use a glass rod to stir for 5 minutes.
Acetone will dissolve the fat.

4) Filter the contents of the acetone and crushed chips into a 100 mL beaker. Make sure all the crushed chips are transferred into the filter paper. Rinse the residue with a further 10 mL of acetone to remove all the fat. Dispose of the filtrate as per teachers instructions. The residue now left in the filter paper should contain the undissolved salt.
Salt does not dissolve in acetone.
Allow the residue to stand until all the acetone has evaporated.


6) Place the filtrate in crucible as shown on the right and heat with a Bunsen burner to evaporate all the water. Be careful that the contents do not boil over. When all the water is evaporated continue heating until the starch appears to be charred. All the starch should now have burnt in atmospheric oxygen.
Allow to cool and add 20 mL of warm water heated in a kettle.

7) Weigh an evaporating dish. Record its mass.
Now filter the contents of the crucible through a filter paper into the evaporating dish.
Check to see if starch is still present by adding one drop of iodine. Iodine should turn black in the presence of starch.

8) Place the filtrate on a hot plate set on low and allow the water to evaporate.
9) After the water has evaporated record the mass of the evaporating dish and the salt.

1)a) Calculate the percentage, by mass, of salt in the chips.
b) How does this compare with the labelling on the packet?
Explain
The following questions are for senior chemistry
2) i. What type of molecule is the starch molecule.
ii. What is it composed of?
iii. What type of reaction forms a starch molecule?
3) i. Why does salt and starch dissolve in water?
ii. What type of bonding takes place between:
- starch and water, use a drawing to best describe this type of bonding.
- salt and water, use a drawing to best describe this type of bonding.
4) Why does salt remain unchanged when heated?
5) What happens to starch when heated?
6) Explain why salt, although highly soluble in water, is not soluble in acetone.
7) How would the final calculation of the salt content be influenced if the test in step 7 was positive for starch?