experiment with lead iodide
Lead nitrate reacts with potassium iodide to form a precipitate known as lead iodide according to the equation below.
Pb(NO3)2 + 2KI => PbI2 + 2KNO3
Follow the appropriate risk assessemnt for lead nitrate and potassium iodide.
10ml pipette
0.1M solutions of lead nitrate and potassium iodide
Funnel
Filling bulb
Distilled water
2 100ml beakers
Filter paper
Scales

Weigh a piece of filter paper.
Pipette 10 mls of lead nitrate solution into a beaker.
Pipette 10 mls of potassium iodide solution into the same beaker and notice the formation of the yellow solid (lead iodide).
Click to see a 120kb video.

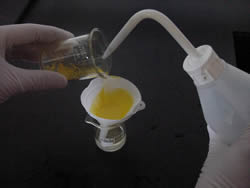
Filter the mixture as shown on the right.
Click to see a 120kb video.

Using distilled water rinse any remaining solid into the filter paper.
Click to see a 120kb video.

Record the results in a table
|
Item |
Mass
(grams) |
|
Filter
paper |
|
|
Filter
paper and lead iodide |
|
|
Mass
of lead iodide |
|
Questions
What is the mass of lead iodide
formed?
Calculate the number of moles of lead iodide formed.
Consider the balanced equation for this reaction
Pb(NO3)2 + 2KI => PbI2 + 2KNO3
For every mole of lead nitrate reacted how many mole of potassium iodide
formed?
How many mole of potassium iodide reacted to form the mass of lead iodide
?
What mass of potassium iodide reacted?
Consider the experiment mentioned below.
Jonathon conducted this experiment. He used a scale that gives readings to two decimal places. He weighed a single piece of filter paper as shown on the right.

He then weighed the same filter
paper with the lead iodide after it had been left to dry overnight.
What is the mass of lead iodide formed?
How many mole of lead iodide is this?
How many mole of lead nitrate reacted?
What mass of lead nitrate reacted?

Item |
Mass
(grams) |
Filter
paper |
|
Filter
paper and lead iodide |
|
Mass
of lead iodide |