1) Evelyn titrates 10.0 ml of 0.100 M potassium permanganate, KMnO4, with 0.100 M oxalic acid, C2H2O4.
The half-equation for the oxidation of oxalic acid in acidic conditions is
![]()
What volume of C2H2O4 should be added to reach the equivalence point?
A. 4.0 ml
B. 10.0 ml
C. 12.0 ml
D. 25.0 ml
Solution

2. Salim is writing a scientific poster to report on his laboratory analysis of a compound.
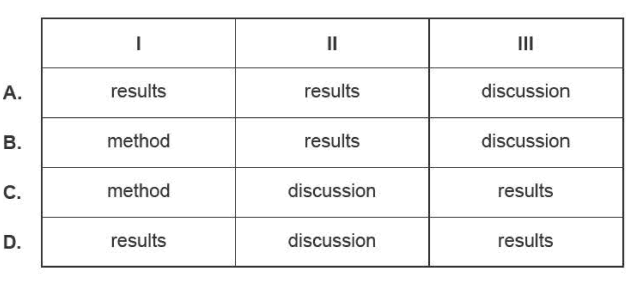
Consider the following three statements.
I. A 50 ml solution of 0.1 M HCI was placed in a conical flask.
II. The average concentration is within the range claimed by the manufacturer.
Ill. The table shows the titres from the titration.
Which section of the poster should include these statements?
Solution
