1.Compound X has a molecular formula of C5H11CIO. The infrared spectrum of Compound X is shown below.
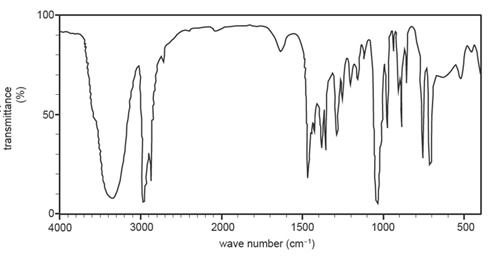
a. Use item 22 of the Data Book to identify the bond type in Compound X that has the highest wave number.
b. The mass spectrum of Compound X, C5H11CIO, is shown below. 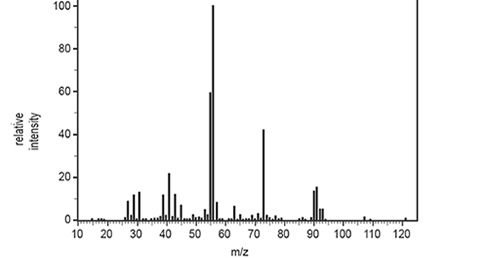
i. Identify the m/z of the base peak. Page 23 of 36 1 mark
ii. Identify the formula of the species that is responsible for the peak at
m/z = 73.
Solution
c. The 13C NMR spectrum for Compound X is shown below. 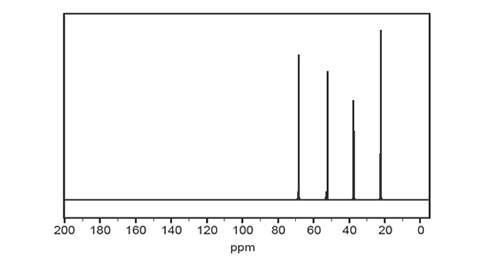
What information do the four peaks in the 13C NMR spectrum provide about the arrangement of the five carbon atoms in the structure of Compound X? Justify your answer. (3 marks)
Solution

d. The high-resolution 1H NMR spectrum for Compound X is shown below.
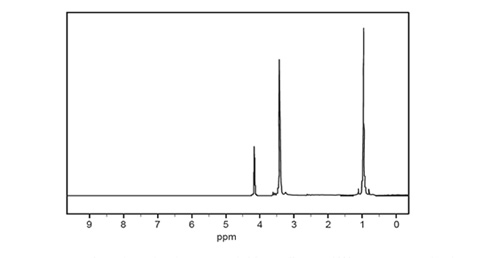
i. Each peak in the 1H NMR spectrum for Compound X is a singlet. Each peak has an associated integration curve. In general, what feature of a 1 H NMR peak is represented by its integration curve?
ii. 1H NMR spectral analysis can provide information about the molecular structure of compounds. In general, what information does the relative size of the integration curves for any given 1H NMR spectrum provide about the molecular structure of the compound analysed?
Solution

The expected integration curves for Compound X are shown in the table below.
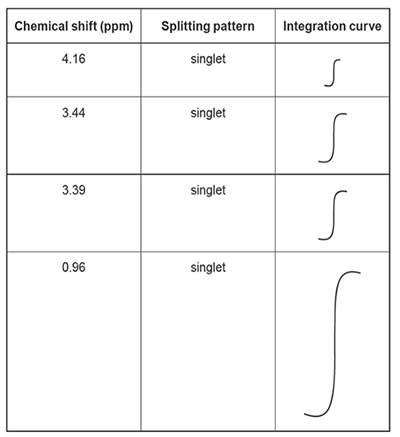
iii. State what the relative size of the integration curves in the table above indicates about the molecular structure of Compound X.
iv. Consider the chemical shift and the size of the integration curve for the peak at 0.96 ppm. Refer to item 24 of the Data Book. What does this information indicate about the structure of Compound X?
Solution

e. In the space provided below, draw the structural diagram of Compound X, using the information provided in parts a-d.
Solution
