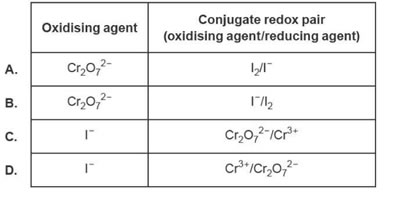
1. Consider the following reaction.
![]()
Which one of the following correctly identifies the oxidising agent and a conjugate redox pair (in the form oxidising agent/reducing agent)?
2. When the galvanic cell, shoiwn below, is operating
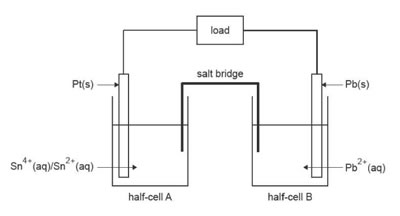
A. total chemical energy is increasing and electrons flow to half-cell A.
B. total chemical energy is increasing and electrons flow to half-cell B.
C. total chemical energy is decreasing and electrons flow to half-cell A
D. total chemical energy is decreasing and electrons flow to half-cell B
Solution

3) Consider the following half-equation.
![]()
A galvanic cell is set up using inert electrodes and the following chemicals:
• half-cell 1: 1.0 M acidified solution of potassium iodate/iodine solution (KI03(aq) / l2(aq))
• half-cell 2: oxygen gas, 02(g), in an alkaline solution.
Which one of the following statements is correct when the galvanic cell is operating?
A. Hydrogen gas is produced at the cathode.
B. The oxidising agent is I03 - and the reducing agent is 02.
C. The concentration of OH- ions decreases at the negative electrode.
D. The mass of the electrode in half-cell 1 decreases.
Solution
4) In a galvanic cell, copper(II) ions, Cu2+, are converted to copper metal, Cu.
What mass of Cu is deposited for each 0.50 mol of electrons transferred in the cell?
A. 1.6 x 10 g
B. 3.2 x 10 g
C. 6.4 x 10 g
D. 1.3 x 102 g
Solution
