1) Photosynthesis is an
A. exothermic redox reaction.
B. endothermic redox reaction.
C. exothermic condensation reaction.
D. endothermic condensation reaction.
2) Which one of the following statements is correct?
A. The oxidation of glucose is reversible in the body.
B. The combustion of glucose provides less energy per gram than the combustion of hydrogen.
C. The oxidation of glucose is given by the equation
6CO2 + 6H2O → C6H12O6 + 602.
D. The combustion of oxygen occurs during cellular respiration.
Solution

3) 0.50 mol of ethane, C2H6 , and 100.0 g of air that is 22.0% m/m oxygen, 02, are injected into a combustion chamber. The equation of the combustion of ethane is
C2H6 (g) + 3½02(g) → 2C02(g) + 3H2O(I).
If complete combustion takes place, which reactant is in excess and by how much?
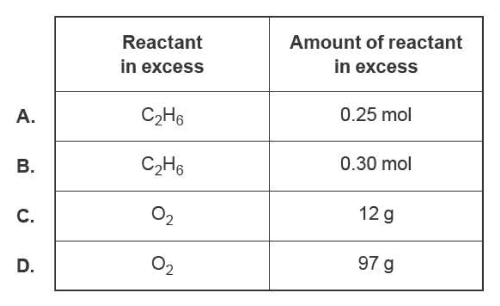
Solution

4) Iron is produced from iron ore using heat and a reducing agent.
Using hydrogen, produced from fossil fuels, as the reducing agent to produce iron is consistent with
A. the concept of a linear economy.
B. the concept of a circular economy.
C. the United Nations Sustainable Development Goal 2: Zero hunger.
D. the United Nations Sustainable Development Goal 11: Sustainable cities and communities.
Solution
5) The combustion reaction between butane gas, C4H10 , and oxygen gas, 02 , is considered irreversible because
A. the forward reaction is exothermic.
B. the products are less stable than the reactants.
C. the rate of the reverse reaction is so slow that it can be ignored.
D. an unlimited supply of oxygen will favour the forward reaction.
Solution

6) Consider the following energy profile diagram for an uncatalysed gaseous reaction. A solid catalyst is
available for this reaction ..
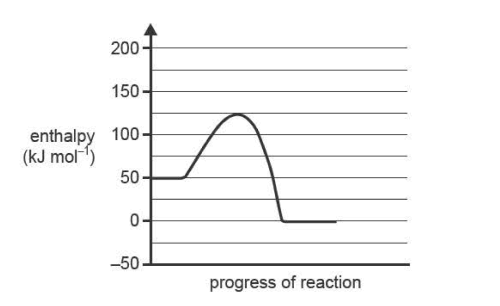
Which one of the following statements is correct?
A. Adding the catalyst to the uncatalysed reaction will increase the energy of the reactants.
B. Removing the catalyst from the catalysed reaction will increase the rate of the reaction.
C. Removing the catalyst from the catalysed reaction will increase the activation energy.
D. Adding the catalyst to the uncatalysed reaction will make the reaction endothermic.
7) Consider the following reaction.
![]()
Which reaction conditions would give the greatest yield of nitrogen dioxide, NO2?
A. 200 kPa and 100 °C
8. 200 kPa and 25 °C
C. 100kPaand100°C
D. 100 kPa and 25 °C
Solution

8) Ammonia gas, NH3(g), can be made by reacting a mixture of nitrogen gas, N2(g), and hydrogen gas, H2(g), using an iron catalyst.
The reaction can be represented by the equation
![]()
The equilibrium constant, Kp, at 400 °C is 1.60 x 10-8 kPa-2.
In a mixture that is at equilibrium at 400 °C, the partial pressure of H2 is 88.7 kPa and the partial pressure of N2 is 43.4 kPa.
a. Write the expression for the equilibrium constant, Kp.
Solution
b. Determine the partial pressure of NH3 , in kPa, in the equilibrium mixture at 400 °C.
Solution
c. At a constant temperature of 400 °C, a change was made to the equilibrium system. Just after the change was made, the reaction quotient, Q, was 1.20 x 10-9 kPa-2.
Identify a change to the system that would result in this reaction quotient. Explain how the system would respond to the change.
3 marks
.
Solution
d. Explain how the iron catalyst addresses the conflict between optimal rate and temperature considerations in the production of NH3 Support your answer with reference to one green chemistry principle. Refer to item 26.ii of the Data Book.
3 marks
Solution

9)
a.
A container with 100.0 g of water was heated using a butane,
C4H10 , gas burner. The following results were obtained.

i. Use item 13 of the Data Book to calculate the amount of energy released by the combustion of C4H10 .
Solution
ii. Calculate the amount of energy absorbed by the 100.0 g of water.
Solution
iii. Calculate the percentage energy efficiency of the system used to heat the water.
Solution

b. Minh is provided with solutions of 0.500 M lead(II) nitrate, Pb(NO3)2(aq), and 0.960 M potassium iodide, Kl(aq), to investigate the reaction below
![]()
Minh calibrated a solution calorimeter and found its calibration factor to be 470.0 J oc-1. Minh performed the reaction above in the calibrated calorimeter.
The initial temperature of each solution was 25.0 °C. Minh stirred the mixture continuously during the reaction.
i. Explain why a calorimeter needs to be calibrated before it can be used to calculate enthalpy changes. 2 marks
Solution
ii. Calculate the maximum temperature that could be reached by the solution in the calorimeter if 0.048 mol of Kl(aq) reacted. 3 marks
Solution
iii. Minh repeated the experiment with the Pb(NO3)2(aq) and Kl(aq) solutions three times in the calibrated calorimeter. The experiment was found to have a high degree of repeatability. The average maximum temperature from the four trials was greater than the expected value.
State one reason that would explain why the average was greater than the expected value. 1 mark
Solution
