1. A piece of blister copper contains 98.5% copper. The blister copper also contains other metals, including
silver, iron and nickel. Electrolysis in an aqueous solution of Cu2+ ions is used to produce 99.99% pure
copper from the blister copper.
In the electrolysis of the blister copper
A. blister copper is used as the cathode.
B. solid iron falls to the bottom of the electrolysis cell.
C. the 99.99% pure copper product should be removed as it forms.
D. the concentration of Cu2+ ions in the electrolyte changes during the electrolysis.
Solution
2. Svante adds a 1 M solution of sodium nitrate, NaNO3(aq), to a beaker. An electric current from a 3 V power supply is then passed between an iron, Fe(s), electrode and a platinum, Pt(s), electrode placed into the solution.
The positive terminal of the power supply is attached to the Fe electrode and the negative terminal of the power supply is attached to the Pt electrode.
Refer to item 1 of the Data Book.
i. State which metal electrode is the anode.
Solution
ii. Write the half-equation for the reaction that occurs at the Pt electrode when the power supply is turned on.
Solution
iii. State why no reaction occurs when the power supply is turned off."
Solution

3. Lithium-ion cells are used in many electronic products.
a. When the cell is recharging, the following reactions occur at the electrodes.
Electrode X: LiCoO2 → CoO2 + Li+ + e-
Electrode Y: 6C + Li+ + e- → LiC6
i. State the polarity of Electrode X during recharging.
Solution
ii. Calculate the mass of LiC6 produced when the cell is recharged using a current of 1.25 A for 1.00 hour.
Solution
b. Artificial photosynthesis can be used to produce hydrogen from water in acidic conditions. A simplified diagram of the acidic artificial photosynthesis process is
shown below.
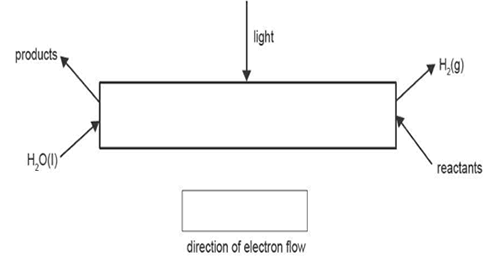
i. Identify the overall energy transformation that occurs during artificial photosynthesis. 1 mark
Solution
ii. In the box shown in the diagram, draw an arrow to indicate the direction of electron flow. 1 mark
Solution
iii. Explain how the electrical charge in artificial photosynthesis processes is balanced given that electrons only flow in one direction. 2 marks
Solution
