1) A triglyceride is reacted with methanol, CH3OH, in the presence of concentrated KOH(aq). The products of this reaction are glycerol and Compound J.
The molecular formula of Compound J is C19H30O2.
What is the molecular formula of the triglyceride?
A. C54H82O3
B. C54H82O5
C. C57H84O3
D. C57H86O6
2) When the triglyceride is reacted with methanol to produce glycerol and Compound J
A. H2O should be added to the KOH directly, because KOH is corrosive and causes skin burns.
B. H2O should not be added to the reaction mixture because it interferes with the desired reaction.
C. H2O should be added to separate the triglyceride from glycerol since the triglyceride is
soluble in water.
D. H2O should not be added to the reaction mixture because it increases the frequency of successful collisions.
Solution

3) A reaction between sulfur atoms in proteins
A. contributes to the quaternary structure through ionic bonding.
B. contributes to the secondary structure through hydrogen bonding.
C. leads to the formation of covalent bonds, and contributes to the tertiary structure.
D. leads to the formation of disulfide linkages, and contributes to the primary structure.
Solution

3) a. Gluten exorphin C is derived from gluten found in bread. The skeletal structure of gluten exorphin C is shown below.
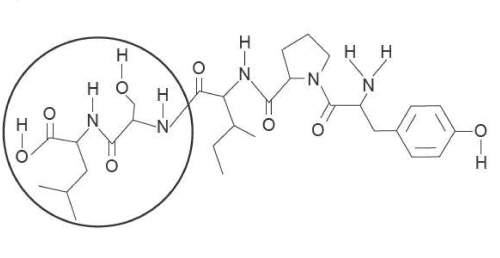
i. Name the bond that joins the monomers in gluten exorphin C.
Solution
ii. Name the type of reaction that results in the formation of the bond between the monomers in gluten exorphin C.
Solution

iii. Use item 20 of the Data Book to name a monomer that reacted to form the circled section of the gluten exorphin C molecule shown above.
Solution
b. The fats in bread can be broken down into compounds including oleic acid and stearic acid. Refer to item 21 of the Data Book.
Page 17 of 36
i. Identify a structural difference between oleic acid and stearic acid.
Solution
ii. Write the reaction for the complete combustion of oleic acid. States are not required. 2 marks
Solution
iii. Predict whether oleic acid or stearic acid would have a higher molar heat of combustion. Explain your answer with reference to item 10 and item 11 of the Data Book. No calculations are required.
Solution

4) A label on a bread packet has the following information.
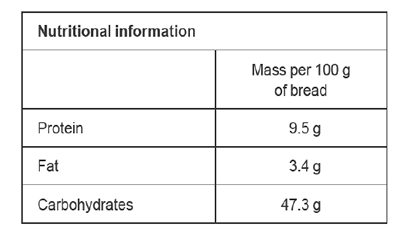
Use item 12 of the Data Book to calculate the energy typically available for the body to use in 100 g of bread.
Solution
