1) The thin layer chromatography plate shown below has a polar stationary phase. It was developed using hexane as the
solvent.
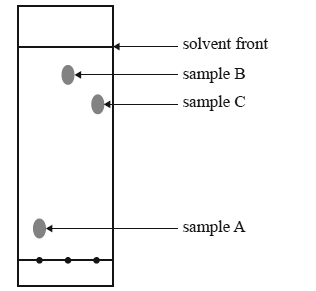
Which sample has the most polar molecules?
A. sample A
B. sample B
C. sample C
D. There is not enough information to determine which sample has the most polar molecules
Solution

3) A forensic chemist tests mud from a crime scene to determine whether the mud contains zinc.
Which one of the following analytical techniques would be best suited to this task?
A. infrared spectroscopy
B. thin layer chromatography
C. atomic absorption spectroscopy
D. nuclear magnetic resonance spectroscopy
Solution
2) High-performance liquid chromatography is used to determine the amount of caffeine in a sample of a soft
drink. The chromatogram below shows the detector response when a standard solution of caffeine with a concentration of
200 mg L–1 is measured using the instrument.

a) What is the retention time of caffeine in this experiment?
Solution

b) On the chromatogram above, sketch the detector response when a commercial soft drink with a caffeine content of
350 mg L–1 is measured using the same instrument.
Solution
