Organic chemistry 2007 VCE
Which of the following statements would apply to compounds that belong to the same homologous series?
I they have similar physical properties
II they have similar chemical properties
III they contain the same functional group
IV they have the same molecular formula but different structures
A. III only
B. IV only
C. II and III only
D. I, II, III and IV

Cuts and wounds are often stitched using a biodegradable polymer with the formula
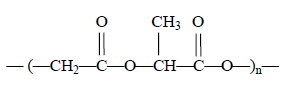
It is made from a condensation polymerisation reaction between lactic acid (HOCH(CH3)COOH) and glycolic
acid.
The formula of glycolic acid is
A. HOCH2COOH
B. HOCH2CH2OH
C. HOOCCH2COOH
D. HOOCCH2CH2OH
Solution

Ethene can be converted into other carbon-containing compounds using the reagents shown in the following
flow chart.
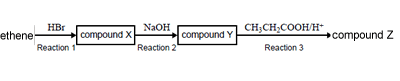
Compounds X, Y and Z are, respectively
A. bromoethane, ethanol, propyl ethanoate.
B. bromoethane, ethanol, ethyl propanoate.
C. bromoethene, ethanoic acid, ethyl propanoate.
D. bromoethene, ethene hydroxide, propyl ethanoate.
Solution
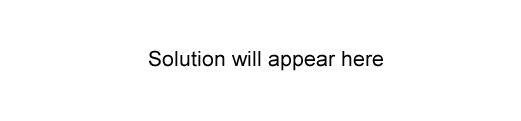
Reactions 1, 2 and 3 can be described as, respectively
A. addition, addition, neutralisation.
B. addition, substitution, condensation.
C. substitution, neutralisation, oxidation.
D. substitution, substitution, condensation.
Solution

The following structure represents the repeating unit of a polymer used in the manufacture of contact lenses.
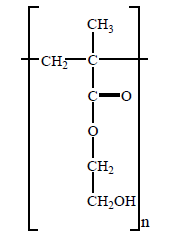
Which one of the following is a correct statement about the monomers that react to form this polymer?
A. Each monomer contains a double bond between carbon atoms which allows addition polymerisation to
take place.
B. Two different monomers react to form the polymer, one with carboxyl groups and the other with hydroxy
groups.
C. The total mass of the monomers is greater than the mass of the polymer formed because water is eliminated
in the polymerisation reaction.
D. Each monomer contains both a carboxyl and a hydroxy group which allows condensation polymerisation
to take place, forming a polyester.
Solution

molecular mass alkanes and alkenes can be produced in the laboratory by heating parafin oil in the presence
of alumina.
a. i. What is the general name given to this process?
Solution
ii. Suggest the likely function of the alumina.
Solution
b. If one of the components of parafin oil is C17H36, write a balanced equation in which this component
forms ethene and only one other product.
Solution
c. A chemist collects three samples of ethene and performs the following reactions. Write balanced equations
for each one.
i. addition of bromine (Br2)
Solution
ii. complete combustion
Solution
iii. heated with steam and a catalyst at 300°C
Solution

A low molecular mass alkane was extracted from a gas sample obtained by this method. The following
sequence of chemical reactions was then performed using this alkane.
The alkane was allowed to react with chlorine in the presence of ultraviolet light. Two compounds, A and B, were formed, each of molar mass 78.5 g mol-1.
One of these two compounds was isolated and allowed to react with potassium hydroxide solution. The product of this reaction was heated with acidified potassium dichromate solution to form C, an acidic
compound.
Draw the structural formulas of compounds A and B, showing all bonds present in the molecules. Give the systematic name of each compound.
Solution

Solution