Properties
of metals
Property
Reason

No-directional forces. Strong forces of attraction remain after the metal is reshaped.
Conducts electricity in both the liquid and solid states.

Delocalised electrons
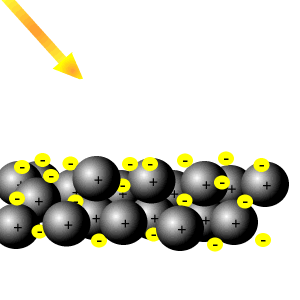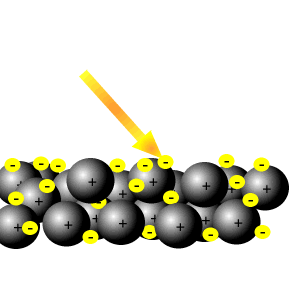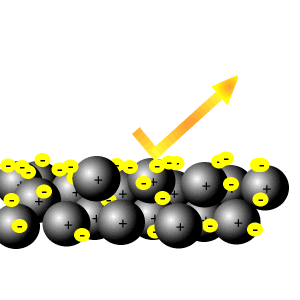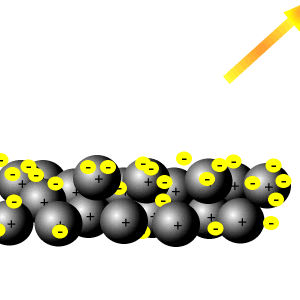
Delocalised electrons help reflect light.
Relatively high melting point. Exception is mercury which exists as a liquid at room temperature.
Strong forces of attraction.

Delocalised electrons help carry energy throughout the metal lattice.