Science of Conflict
Elements and war
The elements known as the halogens ( group 17) of the periodic table are some of the most reactive elements known. They will react with almost everything. Liquid bromine is shown on the left reacting with aluminium metal. Chlorine is another highly reactive halogen.

During the bitter, trench warfare of WW1 many chemicals were used to clear the trenches both by the French and the Germans. Xylyl bromide is a caustic tear gas and was first used against Russian troops. It failed miserably as the Russian winter was so cold that xylyl bromide froze solid.
Surveying the poor results the Germans, in particular Fritz Haber, turned to another element in the same group as bromine, chlorine. Chlorine sits above bromine in the periodic table, and because each chlorine atom is smaller than a bromine atom it is lighter and less likely to freeze. Being above bromine, chlorine is much more reactive and undergoes aggressive reactions with other elements to take one electron.
Chlorine is a highly toxic gas that attacks the skin turning it yellow, green and black and causes cataracts. It also attacks the cells in the lungs causing massive fluid buildup to an extent where the victim drowns in their own body fluid.
Soon soldiers had to fear chlorine based chemicals, such as mustard gas.

After its gas, Germany's most feared weapons were its Big Bertha siege guns. It made more sense to hammer the enemy with metal from its superheavy siege guns than to burn them with bromine and chlorine gas.
These guns weighed 40 tons and were transported in pieces by tractors to assembly points where it took 200 men 6 hours to assemble.
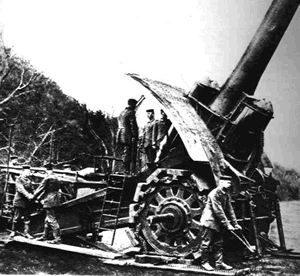
Big bertha siege guns could fire heavy shells at distances that exceeded 16 kilometres in just a few seconds. But this required a great deal of gunpowder which caused problems. The immense heat generated by the gunpowder warped the steel gun barrels. The guns therefore had a life span of just a few days.
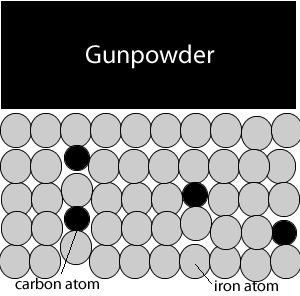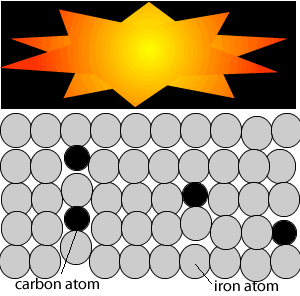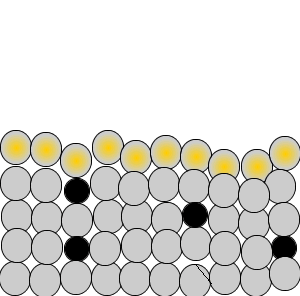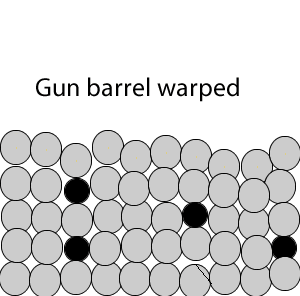
As shown in the animation on the right, iron atoms absorb heat and move past each other. This tends to warp the gun barrel and render it useless. At high temperatures these iron atoms disastrously and spontaneously rearrange themselves resulting in a metal that is brittle, easily cracks and fails.
The Germans came up with the idea of adding molybdenum to the steel. Molybdenum is a heavier and larger atom than iron and can be found in the transition group of metals in the periodic table. It melts at 2623 oC where as iron melts at 1535 oC . Molybdenum atoms, been heavier and larger than iron atoms, get excited more slowly by heat. The fact that molybdenum atom has more electrons means it can absorb more heat than an iron atom.
Now if molybdenum gave steel such desirable properties because of its sheer size may be a bigger atom would give better results. Tungsten was just that metal, placed just below molybdenum on the periodic table it could hold together the iron matrix in steel more strongly than molybdenum. It melts at 3422 oC and has more electrons than molybdenum therefore can absorb more heat. Tungsten was so sought after that the Nazis happily traded looted gold for tungsten.
Being one of the hardest metals known it was used for drill bits that can drill through hard rock or metal and short range missiles known as kinetic energy penetrators that could penetrate heavy tank armour.
A kinetic energy penetrator uses its kinetic energy, which is a function of mass and velocity (1/2mv2), to punch through heavy armour.Once penetrated the heat and metal particle spray generated by the penetrator going through the armor, together with the pressure wave generated by the impact, is enough to destroy the target.
The modern kinetic energy weapon maximises the kinetic energy of the projectile and minimises the area over which it is delivered by:
- being fired with a very high velocity;
- concentrating the force in a small impact area;
- maximising the mass of whatever volume is occupied by the projectile by using the densest metals practical, which is one of the reasons depleted uranium is often used.

2) Uranium tipped weapons are used to easily penetrate heavy armor. Explain why with relevance to the periodic table.

Chlorine, iodine, fluorine, bromine.
How would you expect iodine gas to behave?