Science of Conflict
Chemistry of explosive reactions
All chemical reactions obey the "Law of Conservation of Mass". This law states that mass can not be created nor can it be destroyed during a chemical reaction, the total mass of the reactants equals the total mass of the products. Yet an explosion is a chemical reaction which appears to produce more substance than was originally there. This is impossible and the illusion that more mass is created during the explosion than was originally present is explained below.
Explosive reactions occur quickly, produce mainly gases and release a great deal of heat. Heat energy produced by the reaction makes the gases expand with incredible speed and destructive force.
Take trinitrotoluene (TNT) (C7H5N3O6) for example, it quickly decomposes to form a number of gas molecules and a great deal of heat according to the equation below.
2C7H5N3O6(s) => 3N2(g) + 5H2O(g) + 7CO(g) + 7C(s) + energy
For every two TNT molecules we produce 15 gas molecules. The solid carbon produced is usually seen as black dust that remains after the explosion.

When you look at a chemical equation the number of atoms of each element is the same before and after the reaction, they have just being rearranged. This is because mass is conserved, that is, no atoms are destroyed or created during chemical reactions.
Take the explosive reaction between hydrogen and oxygen for example. It is shown on the right as a balanced chemical equation. You can see that the same number of hydrogen atoms are on the left and right as are the oxygen atoms, they are just rearranged to form new products.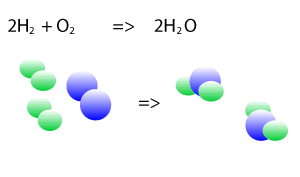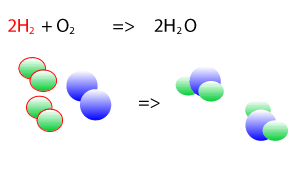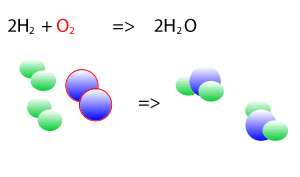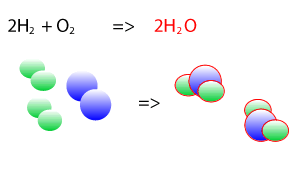
Surface area is another consideration, the greater the surface area of the reactants the greater the speed of reaction. This is because for a reaction to occur the reactant molecules must collide together. So the greater the surface area exposed to collisions the quicker the reactants react.
Heat also increase the rate at which a reaction takes place. So the more heat that is produced by the reaction the faster the rate at which it proceeds. This is because heat makes all molecules move faster, especially gas molecules.
Click to see a quick and easy demonstration performed in a fume cupboard that will demonstrate the relationship between surface area and rate of reaction.
Mixing the reactants well will also increase the rate of reaction as this will also facilitate collisions between reactants. When reactants are mixed together they must be able to find each other and collide together in order to react. Thorough mixing ensures that this will happen in the shortest possible time.
This can be demonstrated by the following demonstrations , the thermite reaction and the burning sugar. When conducting these two reactions it will be obvious what impact poor mixing of the reactants has on the rate of reaction.
1)
a) Does the reaction on the right obey the law of conservation of mass? Explain
b) C3H8 + O2 => CO2 + 3H2O
i) How many carbon atoms appear on the right ?
How many appear on the left
ii) How many hydrogen atoms appear on the right ?
How many appear on the left
iii) How many oxygen atoms appear on the right ?
How many appear on the left
iv) Is the law of conservation of mass obeyed here? Explain
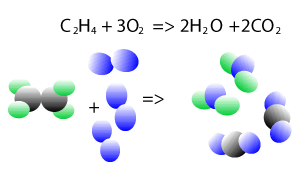
4) Why does the gas coming from the hot plate not explode when it is ignited with a match?

