Magic of Science
Rising water trick
For this trick you will need a candle, matches, some food dye, a bowl and a long slim glass.
Place the the candle in the bowl with the coloured water as shown.
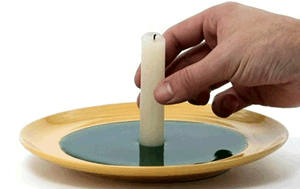
Now light the candle and place the glass over it.
Watch what happens.
Explain what you see. Form a hypothesis to explain how the water is forced up the glass
You may wish to consider the following questions before you attempt an explanation.
Roughly what percentage of the air is due to oxygen?
What is produced when the candle burns?
What happens when gas is heated ?
What is the best explanation for your answer above?
Why does the flame become extinguished?

Wax is composed of hydrocarbon molecules that are, on average,25 carbons long. The general formula for wax can be described simply, for our purposes, as C25H52 So we can write the equation below
2C25H52(s) +76O2(g) => 52H2O(g) + 50CO2(g)
One suggestions to explain how the water rises up the glass is that there are less molecules in the air inside the glass after the candle goes out than before. This creates a partial vacuum that sucks the water up the glass. However, according to the equation above there appears to be more gas molecules present after the reaction than before.
How many gas molecules are used to burn 2 molecules of wax?
How many gas molecules are produced when two wax molecules burn?
This reaction will produce
Suggest what might happen to the water vapour molecules after the candle goes out.
Suggest what might happen to the carbon dioxide gas molecules. Design an investigation to prove this. Hint You may use universal indicator.
What is your best explanation for the rising water trick?