Periodic table
Position, Position, Position

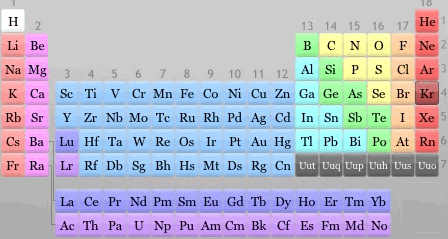
The periodic table is a place where all the known elements of the universe are placed in unique neighborhoods according to their behaviour. With the periodic table, an element can always talk about position, position, position.
You see, if you are an element lucky enough to be in:
-
column one, known as group 1, you will have a fiery relationship with water. Contact with water will cause all period 1 metals to react violently and produce explosive hydrogen gas. The further down the group we go the greater the violence when they react with water. Click to see there reactions with water
- column 18, known as the noble gases. These substances exist in the gaseous state and are the bachelor elements. They hardly react with any other element, happy to stay as they are, single. This is why helium and not hydrogen gas is used in dirigibles.
The group of elements that form the middle of the periodic table are known as the transition elements. Elements in this group are metals with a varied history. We have the working class hero, iron (Fe) in amongst some very celebrated metals such as gold (Au), silver (Ag) and platinum (Pt). These metal elements are also used to produce coloured glass.

Transition metals are often used in fireworks to generate colour. Copper is used to generate blues, while a mixture of copper and strontium will generate purple and strontium on its own will give a deep red colour.

But how are the elements placed in their neighborhoods on the periodic table?
It is all to do with electronic structure.
The atom is composed of a nucleus, made of protons and neutrons, and a number of particles with negative charge known as electrons that orbit around the nucleus in specific orbits. The first orbit, known as the n=1, can hold 2 electrons, the second can hold 8, the third 18 and the forth 32. It is the way that electrons occupy the energy levels of the atom that places the atom in a unique position in a periodic table neighborhood.
The atomic number of an atom is the number of protons in its nucleus. The first ten elements are shown below. The atomic number identifies the element. For example, all atoms found with 2 protons in their nucleus will be identified as helium.
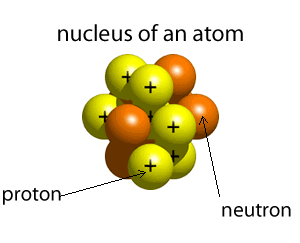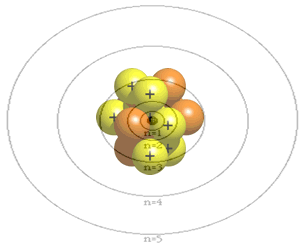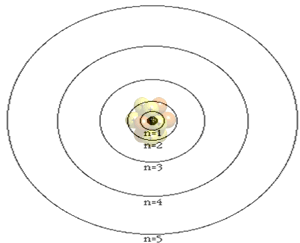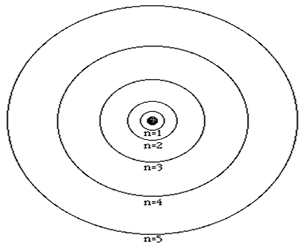
Click
to see how the electrons are arranged in the atoms below.
Hydrogen atomic number 1
Helium atomic number 2
Lithium atomic number 3
Beryllium atomic number 4
Boron atomic number 5
Carbon atomic number 6
Nitrogen atomic number 7
Oxygen atomic number 8
Fluorine atomic number 9
Neon atomic number 10
Sodium atomic number 11
Potassium atomic number 21

Have a look at the atoms in particular groups.
Lets take group 1
Lithium
Sodium
Potassium
Group 2
Beryllium
Magnesium
Calcium
Group 17
Fluorine
Chlorine
Group 18
Helium
Neon
Argon

1) Some science magic shows use sodium, as shown on the right, to demonstrate the reaction between water and exploding metals.
What other metal can be used:
- to create a greater explosion?
- to create a smaller explosion?
2) Which statement best describes protons?
3) Which statement best describes neutrons?
3) Which statement best describes electrons?

4) An neutral atom has 12 protons in its nucleus
5) Look at the electron arrangement of the elements in group 1 of the periodic table.
i) Can you identify any pattern?
ii) What can you tell me about the element found in group 1, row 3?
How does this element differ from the one above it, in terms of electron arrangement?
iii) What determines how elements behave in nature?
6)
Since sodium is very reactive it must be kept away from other substances such as water or air. Sodium is best kept in a container with
7) Explain why helium is used rather than hydrogen to inflate lighter-than-air vehicles?