|
Black inks
|
| Draw
a line in pencil 1cm from the bottom of the chromatography paper.
|
 |
| Place
a thick dot of ink at the centre of this line. |
 |
| Select
a beaker and pour in solvent liquid (methylated spirits) to a depth of 0.5cms.
Place the chromatography paper into the beaker taking care not to submerge
the dot in the solvent. |
 |
| A
number of chromatograms can be developed in one beaker as shown on the right. |
 |
|
The chromatorgram formed is
unique for each type of ink. The individual dye components can be identified
by their unique Rf values. The Rf value can be calculated
as shown below.
Rf = distance travelled
by the solvent("A") / distance travelled by the dye("B").
|
|
|
Example: The chromatogram of a black ink is shown on the right. Calculate
the Rf value
for the purple dye.
The distance travelled
by the solvent("A") is 8 cm while the distance travelled by
the purple dye("B") is 7 cm.
Rf = 7/8
= 0.87
|
|
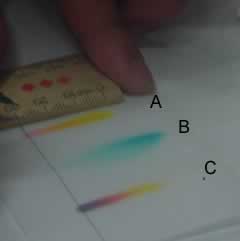 |
The
chromatograms formed can be very spectacular and provide a unique fingerprint
for the type of ink used. Chromatogram "A" is that of an orange
coloured ink while "B" is of a blue ink and "C" is the
chromatogram of a black ink. |
A
letter was forged with black ink. Chromatograms revealed that the ink from
the authentic letter did not match that of the forgery. Five suspects were
arrested and samples of black ink taken from their premises and examined.
View the results
and decide which suspect needs to be questioned in more detail.
One of the five suspects claims to be the author of the original letter.
Is any suspect likely to be the author? Explain. |
|
|
|
Continue
with an exercise on plant pigments
|








