|
Molecular Spectroscopy |
|
| All matter gives a unique pattern when subjected to this technique. Light energy (Ultra violet) is absorbed by different molecules in various ways. The atoms in the molecule will absorb energy and vibrate, rotate and increase the energy of their electrons at different rates. | |
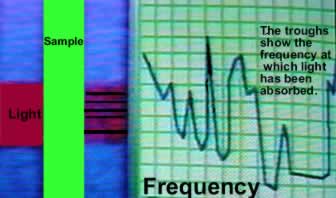 |
By measuring the different frequencies of light that are absorbed by the molecule and plotting the results we get a spectrum such as the one on the left. An instrument called a spectroscope produces this unique pattern (spectrum) of each molecule and as such is said to be the molecule's fingerprint. This technique is used to detect drugs and is very sensitive. Computers are used to match the patterns of the samples to known patterns in the data base. |