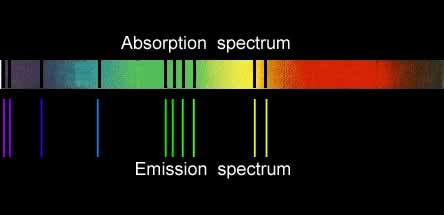
Absorption
spectroscopy exercises
Which of the three elements are present in the cloud.
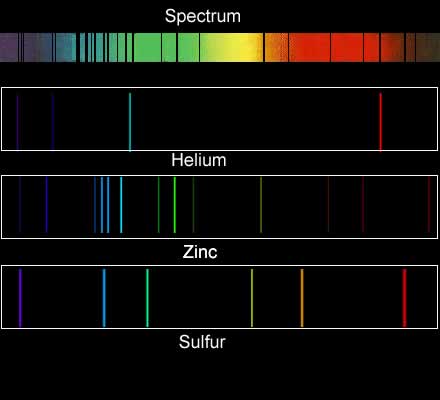
Below are the
emission and absorption spectra of copper. One is a series of black lines
on a coloured background while the other is a set of coloured lines on
black background.
Account for the difference in appearance of the two spectra.
Account for the identical positioning of the lines in the two spectra.
Account for the difference in appearance of the two spectra.
Account for the identical positioning of the lines in the two spectra.