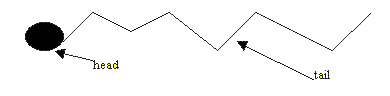
Detergents are big molecules that make it easy for water and oil to mix. Water and oil do not mix and the water molecules can not come close to the grease particle to dissolve it. The detergent molecule allows the water to come really close to the grease particles and dissolve them. To do this the detergent molecule has two parts to it, a water attracting part (head) and a long oil loving tail. Detergent molecules also act to lower the surface tension of water. Water tension is the surface energy of water. The water molecules are held together with a certain amount of energy. Detergents act to decrease this amount of energy. To see a 120kb movie click here.
Notice how the water molecules now pull apart (taking the oil droplet with it) as the detergent is added. The force between the water molecules is decreased.

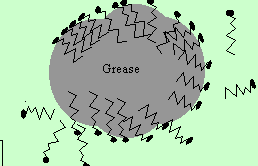
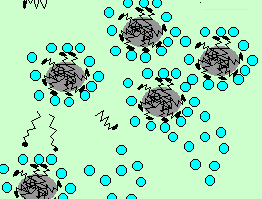
The water now moves closer to the small grease particles and surrounds them. The small grease particles, covered in detergent molecules, now dissolve in the water.
Detergents have been around for many thousands of years. The early cave man found its uses when animal fat fell into the ashes of the fire to create the first primitive soap.
You can make soap using animal fat and caustic soda.