Selecting an indicator
reference to http://www.chemguide.co.uk/physical/acidbaseeqia/indicators.html
An indicator is a chemical compound, usually an acid, whose conjugate base has a different colour to the acid. Each indicator has a specific pH range in which it will change colour and are used to indicate the end point of a titration.
Remember that the equivalence point of a titration is where you have mixed the two substances in the exact stoichiometric ratio given by the balanced equation. So we need to choose an indicator which changes colour as close as possible to that equivalence point. The equivalence point, as you will see, changes depending on the strength of the acid and base titrated..
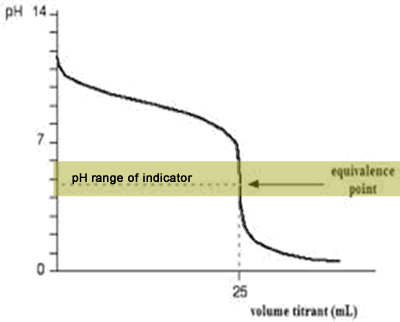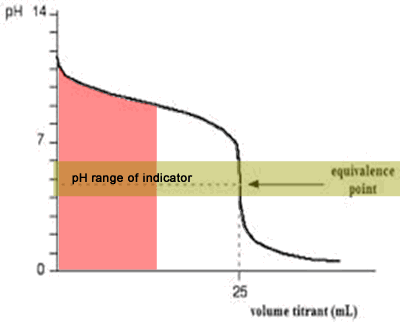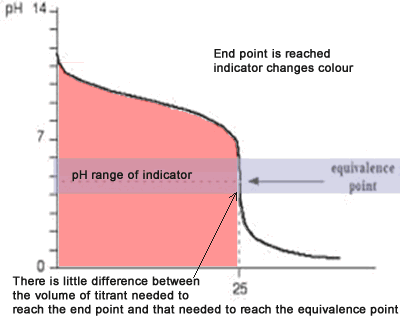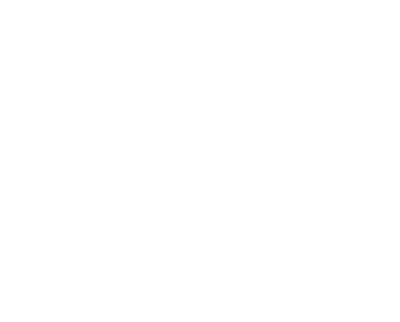
The diagram on the right shows the pH curve for adding a strong acid to a strong base with the pH ranges for methyl orange and phenolphthalein also shown. You can see that neither indicator changes colour at the equivalence point.
However, the graph is so steep at that point that there will be virtually no difference in the volume of acid added whichever indicator you choose. However, it would make sense to titrate to the best possible colour with each indicator. For example, with phenolpthalein you would titrate until it turns from pink to clear and with methyl orange until an orange colour is noticed. The colour change of methyl orange is harder to detect as it changes from yellow to orange.
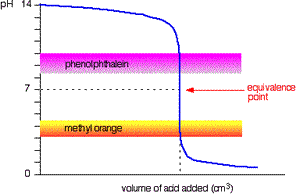
Titration of strong base and strong acid.
This time it is obvious that phenolphthalein would be completely useless. However, methyl orange starts to change from yellow towards orange very close to the equivalence point.
You have to choose an indicator which changes colour on the steep bit of the curve.
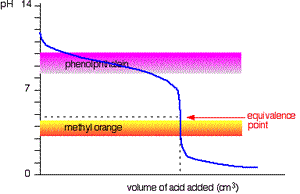
Titration of weak base and strong acid.
When titrating a weak acid with a strong base the methyl orange is useless. However, the phenolphthalein changes colour exactly where you want it to
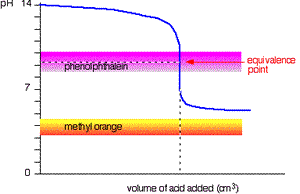
Titration of strong base and weak acid.
The curve on the right is for a case where the acid and base are both equally weak such as, ethanoic acid and ammonia solution. It is obvious that both indicators are useless. The pH curve changes gradually and lacks a sharp change. Phenolphthalein will have finished changing well before the equivalence point, and methyl orange falls off the graph altogether.
This tells us that you would never titrate a weak acid and a weak base in the presence of an indicator but rather use a pH meter.
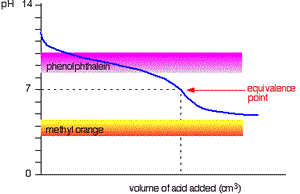
Titration of weak base and weak acid.
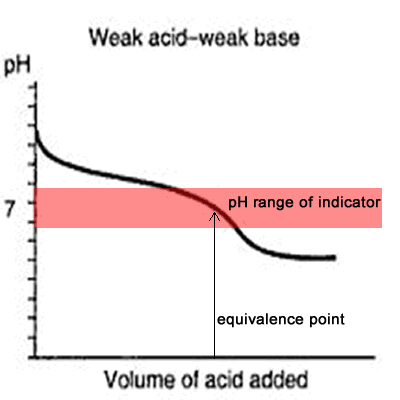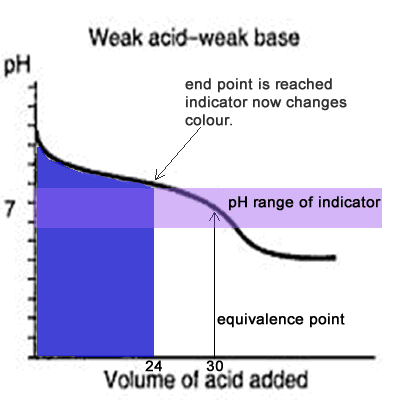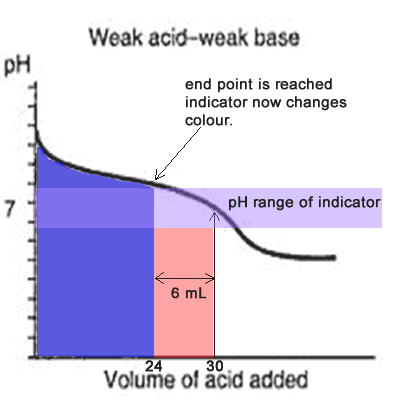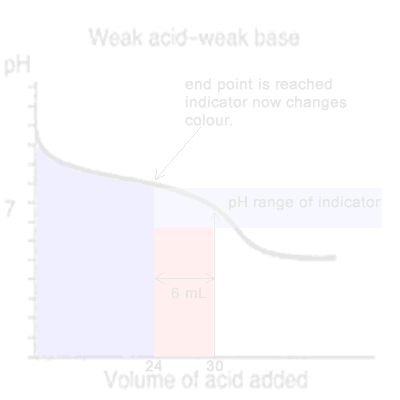
Since the titration curve of a weak acid against a weak base has no steep section it is difficult for an indicator to detect the equivalence point with any certainty. Even if the equivalence point falls within the pH range of the indicator it is still vary unreliable, as the animation on the left shows.
This is why we use back titration to determine the end point of a tritration of weak acid or base.
Say for example we were titrating a solution of NH4+ ions with a standard solution of Na2CO3 . This is a titration of weak acid and a weak base and the titration curve has no steep section.
So we add excess Na2CO3 solution and then back titrate with a strong acid, such as HCl, to determine the excess Na2CO3 remaining and hence how much reacted with the ammonium ions.
The steepness of the titration curve between the weak base and strong acid now allows for an accurate determination of the end point, as shown on the left.