The aim of this activity is to calculate the approximate volume of one atom of iron.
We
will assume that their is no space between each iron atom packed tightly
to form the nail. We will also assume that there are no impurities in
the nail.
Weigh a large iron nail.
We record a mass of 12.87 grams
Calculate the number of iron atoms that a mass of 12.87 represents.
Click to see how this is done.

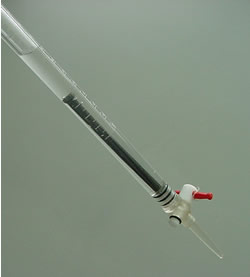
Record the water level before the nail is placed int he burette. Keep in mind that the scale on the burette is read backwards. The next reading will be lower than the first.
The reading is 44.10

Now record the volume of water after the nail is placed into the burette and submerged.
The reading is 42.40
The difference between the two volumes represents the volume of the iron nail.
The nail has a volume of 1.7 cm3

- 1.70 cm3 / 1.38 X 1023 atoms
- 1.23 X 10-23 cm3
|
Atom
|
Volume
|
| Copper | |
| Aluminium | |
| Lead |