|
Titration Analysis of solutions Titration is used to analyse the amount of solute
present in a certain volume of solution. One solution is mixed with
another and compared. |
 |
|
 |
A burette is used to deliver variable, accurate, volumes of solution. It is a very accurate instrument that can give readings to 2 decimal places. Preparing the burette. - wash the burette with the liquid that the burette is to be filled with. - make sure the burette is fixed upright on the stand. - using a funnel, fill the burette - record the level of the liquid. Mixing the solutions together. - Using a pipette, deliver a very accurate volume of the liquid you wish to test into a 50 conical flask. - Place one drop of indicator into the flask with the liquid. - Place the flask under the burette outlet. Open the outlet and deliver liquid from the burette into the flask. With one hand you control the tap on the burette and with the other you constantly swirl the flask. |
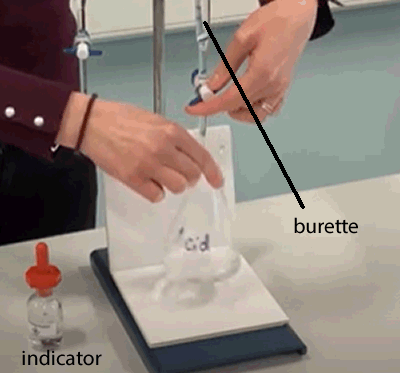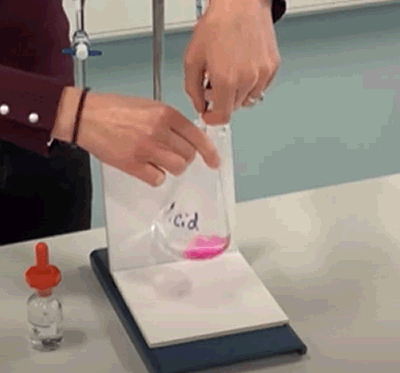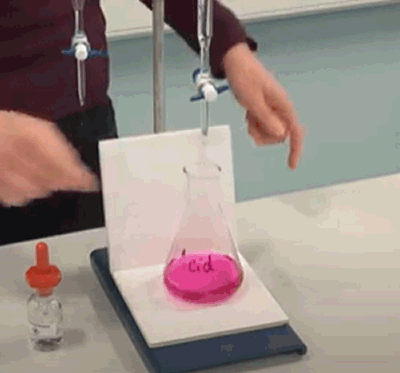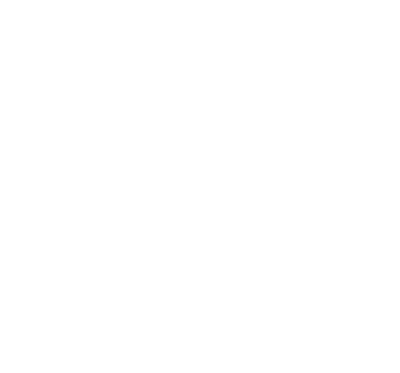
Often the colour change will disappear after swirling for a few seconds. This indicates that you are very close to the end point. |
- Liquid from the burette is added until a permanent colour change, as shown on the right, is achieved by the indicator in the flask. Often the colour will disappear after swirling. This often indicates that the end is probably a drop or two away. - Record the level of the liquid in the burette and calculate the amount of liquid that was added. Repeat the steps above several times. The first time it is done quickly so we can get a rough idea as to how much liquid is needed. The second, third and forth times the liquid is added more carefully until the last drop actually changes the colour of the indicator permanently. |
||
View the video on the right.
|
||
Consider the video on the right. It shows how a solution can be diluted using a volumetric flask and pipette.
|
|
|
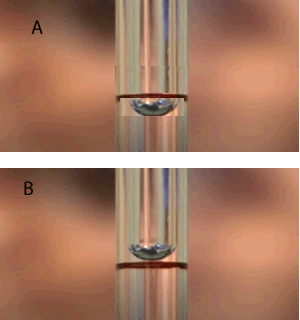
1. Which one of the two images represents the correct level of the solution when filling the pipette? 2. Which statements below are True? i. Always use the filling bulb to push out all the liquid from the pipette. ii. Place any unused solution of the original sample back in its original container. iii. When a filling bulb is not available you can pipette by mouth with extreme caution. iv. A 25 mL pipette can be used to deliver 20 mL of solution if the user is careful and precise in filling the pipette to the appropriate level. |
 |
|
| Conduct a titration activity | ||