Methanol can be produced in a reaction between carbon monoxide and hydrogen according to the following
equation.
CO(g) + 2H2(g) => CH3OH(g); ΔH = 90 kJ/ mol
Which one of the following changes would occur when a catalyst is added to an equilibrium mixture of carbon
monoxide, hydrogen and methanol?
A. The value of ΔH would increase.
B. The amount of methanol would increase.
C. The temperature of the surroundings would increase.
D. The rates of both the forward and reverse reactions would increase.
Solution
Which statement about the behaviour of a catalyst in this reaction is correct?
A. It decreases the activation energy of the forward reaction only.
B. It increases the activation energy of the forward reaction only.
C. It decreases the activation energies of both the forward and back reactions.
D. It increases the activation energies of both the forward and back reactions.
Solution

Consider the following information for the reaction
A + B → C.
heat of reaction -120 kJ/ mol
activation energy +200 kJ/ mol
The activation energy, in kJ /mol, for the reaction
C → A + B, is
A. -320
B.- 80
C. +80
D. +320
Solution

The calibration factor of a calorimeter can be determined by performing in the calorimeter a reaction which produces a known quantity of energy, and measuring the rise in temperature.
In one experiment, 50.0 mL of 0.400 M lead nitrate was added to 50.0 mL of 0.760 M potassium iodide in a solution calorimeter encased in a polystyrene insulating jacket. The mixture was stirred continuously and the temperature rose from 18.42°C to 20.50°C as the following reaction occurred.
Pb(NO3)2(aq) + 2KI(aq) → PbI2(s) + 2KNO3(aq); ΔH = -49.0 kJ mol-1
a.Calculate the amount, in mole, of lead nitrate.
Solution
b. Calculate the amount, in mole, of potassium iodide.
Solution
c. Calculate the energy released by the reaction in the calorimeter, in J.
Solution
d. Calculate, to an appropriate number of significant figures, the calibration factor of the calorimeter and its contents, in J°C-1 .
Solution
Two errors that might have occurred during this experiment are listed below. For each error, indicate the likely effect on the calculated calorimeter factor by placing a tick in the appropriate box.
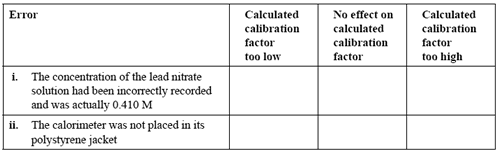
Solution
Give an explanation for your answer to part ii. above.
Solution
