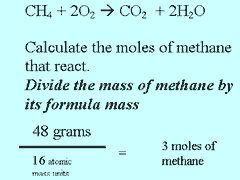
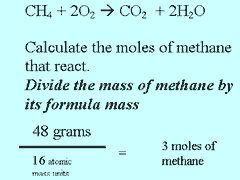
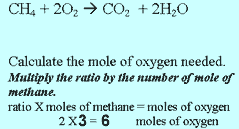
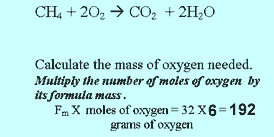
|
Mass-mass stoichiometry |
|
Methane burns in oxygen to produce carbon
dioxide and water according to the equation above. Step 1 Calculate the number of mole of methane present.
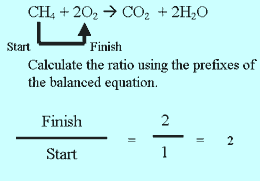
Step 2 Calculate the ratio from the prefixes in the balanced equation above
Step 3 Calculate the number of mole of oxygen used.
Step 4 Calculate the mass of oxygen used.
|