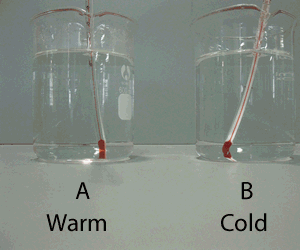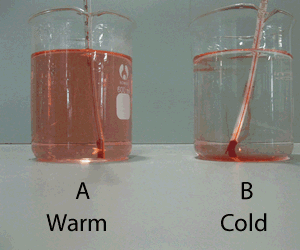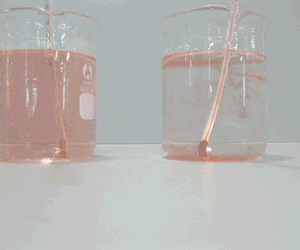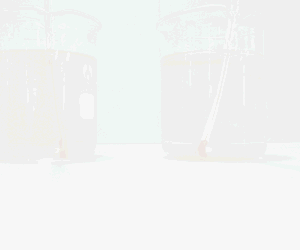
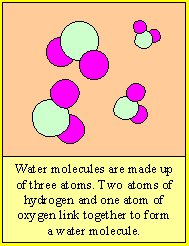
Molecules are small particles of matter made up of more than one atom. Water for example is made up water molecules. Each water molecule is made up of two atoms of hydrogen and one atom of oxygen.
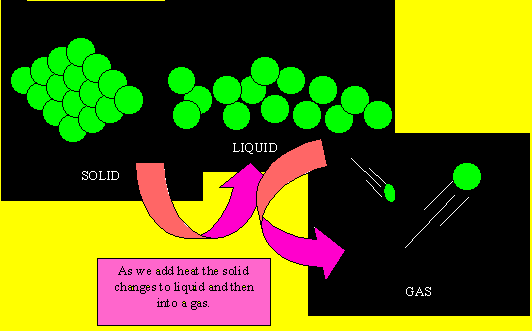
Solids have their small particles firmly held in position. The particles (be they atoms or molecules) do not move very much but simply vibrate in position. As they gain energy, in the form of heat, they start to move and slide past each other. When this happens the substance is said to be a liquid. Now as more heat is applied the particles move very quickly and are like bullets flying through space. At this point the substance is said to be a gas. When a gas is cooled the particles lose their energy and come closer together to form a liquid. When cooled further, the particles lose most of their energy and become fixed into position and simply vibrate on the spot.
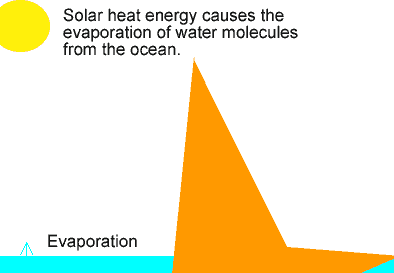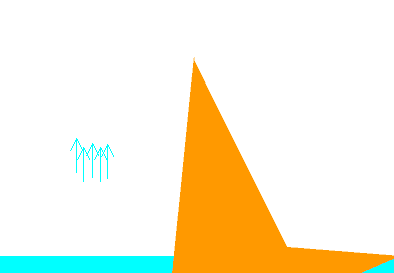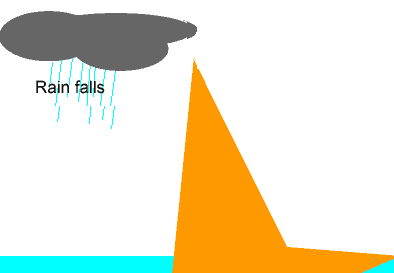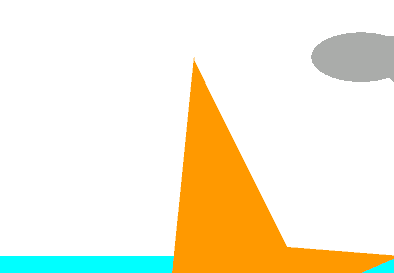
The animation on the left shows the different stages of the water cycle. Explain the properties of the water particles as they:
-evaporate from the ocean;
-condense to form a cloud;
-form ice.