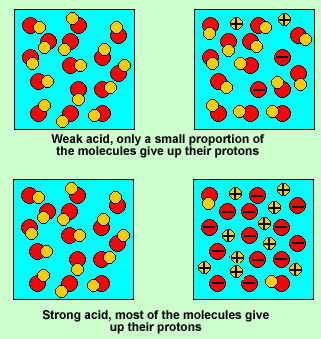
Strong acid or concentrated acid
A strong acid molecule will readily give up its proton to a water molecule present in the solution. Weak acids do not give their protons to the water molecule easily.
However when forced to react with a base most weak acids readily give up their protons to the base. So when we react, as we do in titration reactions, a solution of weak acid, such as vinegar, with a base, all the vinegar(acetic acid) molecules will give up their proton.
Jane was given two 20.0 ml acid solutions. Apparently one was of an unknown weak acid and the other was of sulfuric acid (a strong acid used in car batteries).
She was asked to identify the strong acid by titration with a base of accurately known concentration. Is this possible? Why?