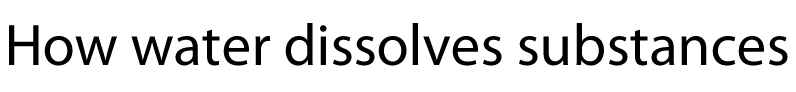
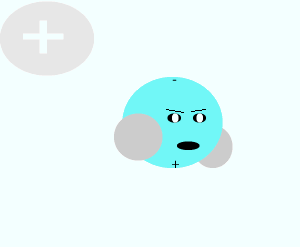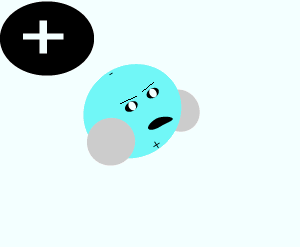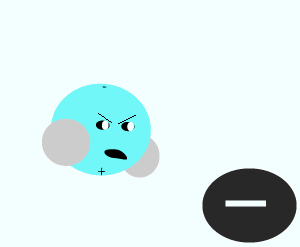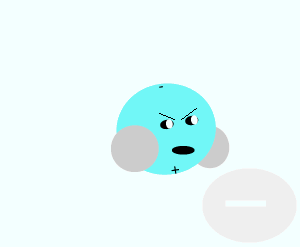

Salt crystals are made up of ions. The water molecules are attracted to the individual ions forming the crystal. As water molecules approach the crystal they surround each ion and separate it from the crystal lattice. Individual salt particles now surrounded by water molecules float freely in the water. A salt solution is formed. Solutions of ionic substances can conduct electricity due to the fact that ions are now free to move throughout the solution.
Not all ionic compounds are soluble.

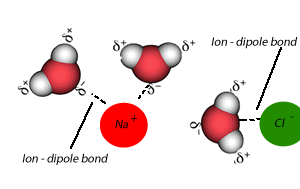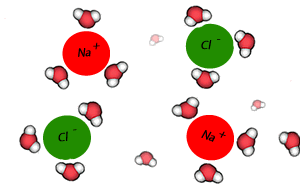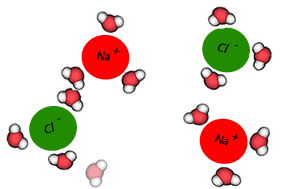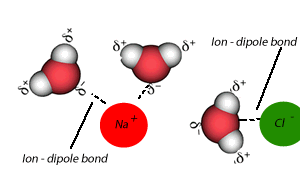
A liquid that is used to dissolve another substance is given the general term of solvent, while the substance being dissolved is known as the solute. In this case salt is the solute and water is the solvent. Notice the formation of ion-dipole bonds between the ions and water molecules.