Evaporation
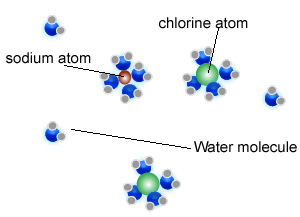



1) a) Describe how the water molecules dissolve the salt crystal.
b) Why does the salt reappear when water evaporates?
2) What is the difference between a soluble and an insoluble substance?
3) Why can we not see the dissolved salt particles in the water but we can see the solid salt crystals?
4) Sugar is a soluble substance. Explain what happens to a cube of sugar as soon as it is placed in water.
5) Soluble nutrients can be absorbed by the roots of plants, through microscopic holes, where as insoluble nutrients can not. Explain why.