There are many ways to express the concentration of a solution. For very small concentrations, such as those encountered with safe limits of heavy metals, we use the unit ppm (parts per million). This unit of concentration can be expressed a number of ways but the true definition of ppm is shown on the right.
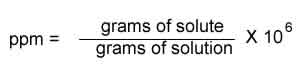

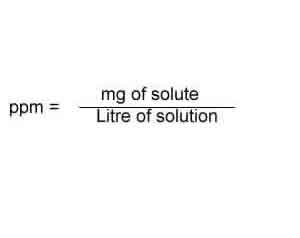
Or the number of milligrams of solute in a Kg of solution.
In other words it is one part, be it grams or millilitres, of solute to one million parts of solution.

Lead and mercury contamination of fish is often measured at these low levels of ppm.

3.2 X 10-2 grams of mercury were found in 2.0 litres of sea water near an industrial plant. Express the concentration of mercury in ppm.
Solution
The meat from freshly caught fish was analysed and found to contain lead at a concentration of 45 ppm. What mass of lead is present in 320 g of fish meat?
Solution
Oxygen gas is found to exist in a sample of sea water at 450 ppm. Convert this to mol/litre.