
Hide the solution

Hide the solution
Solution
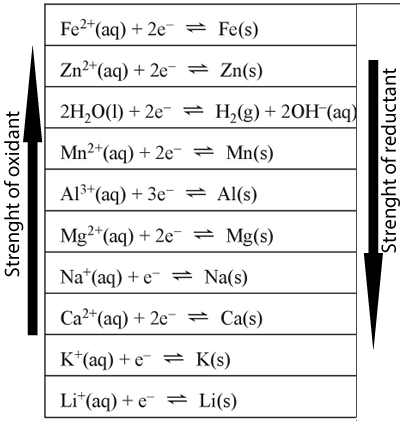
Aluminium metal is the only reductant present . It will react with an oxidant that is above it on the electrochemical series. In fact it will react with the strongest oxidant present. A metal will be deposited in the iron(II)nitrate solution only. The iron(II) ion is higher than magnesium and will reacti spontaneously to form solid iron metal.
When the student dipped the magnesium metal in the calcium nitrate solution a reaction was ovbserved to take place. Once again magnesium will react with the strongest oxidant present that is above it on the electrochemical series. The only oxidant present that is above magnesium is water. The overall reaction is shown below.
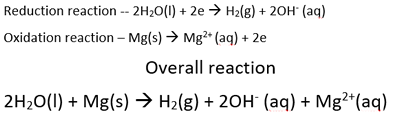
The student then mixed together the solutions of calcium nitrate and potassium nitrate and placed the magnesium in the resulting solution. She noticed a reaction taking place. Write the overall equation for the reaction taking place.
The only oxidant above magnesium, is water.
So the overall reaction is
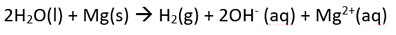
Hide the solution
|
Electrical energy Oxidising and reducing strengths. |
 |
|
For each of the situations below write the oxidation, reduction and overall reactions. 1) Aluminium nitrate is added
to a solution of potassium nitrate and a solid piece of magnesium added. 2) Calcium metal is placed in a solution of aluminium nitrate and magnesium nitrate. 3) Sodium metal is placed in a solution of aluminium nitrate. 4) Iron(II) nitrate and zinc nitrate solutions are mixed and a piece of aluminium metal placed in the resulting solution. 5) A piece of aluminium metal is placed in a iron(II) sulfate solution. a) Identify the oxidant and the reductant b) Write the oxidation and reduction reactions. c) Write the overall reaction
occuring in the solution. 6) A student has four solutions
and a piece of magnesium metal. The solutions are : iron(II)nitrate,
sodium nitrate, calcium nitrate and potassium nitrate. b) When the student dipped the magnesium in the calcium nitrate solution a reaction took place. Write an overall equation for the reaction that occured. c) The student then mixed together the solutions of calcium nitrate and potassium nitrate and placed the magnesium in the resulting solution. She noticed a reaction taking place. Write the overall equation for the reaction taking place. Solution
|