Electrical energy
Oxidising and reducing strengths example.
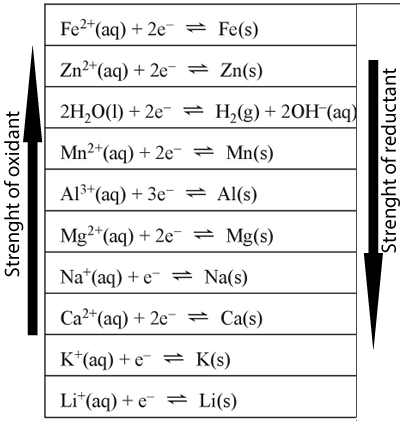
A piece of aluminium metal is placed in a solution containing iron(II)nitrate
Step 1) Identify all the oxidants
present
![]() ,and
,and ![]()
Step 2) Identify all the reductants
Al
Step3) Select the strongest
oxidant and write the half equation as per series.
The strongest oxidant is ![]() and
the equation as per series is
and
the equation as per series is ![]()
Step 4) Select the strongest
reductant and reverse the equation.
The strongest reductant is Al and the equation reversed is ![]()
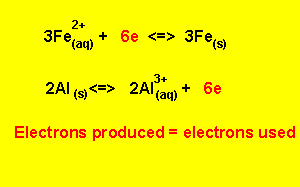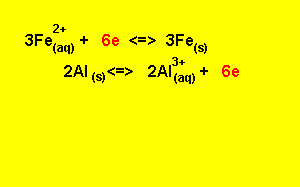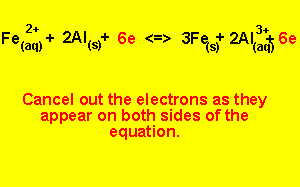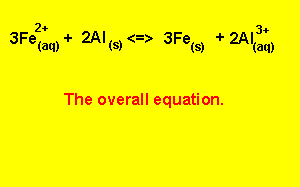
Step 5) To write the overall equation we must first make sure that the number of electrons produced during oxidation is the same as the number produced during reduction. In this example three electrons are produced and two electrons are used, the number of electrons used must equal the number of electrons used.
We find the lowest common multiple of 2 and 3 (which is 6) and multiply each equation by the appropriate number to achieve 6 electrons
3
X (![]() )
)
2
X ( ![]() )
)
Follow the animation below